Characterization of the Oxidative Stress in Renal Ischemia/Reperfusion-Induced Cardiorenal Syndrome Type 3
- PMID: 33102574
- PMCID: PMC7568802
- DOI: 10.1155/2020/1605358
Characterization of the Oxidative Stress in Renal Ischemia/Reperfusion-Induced Cardiorenal Syndrome Type 3
Abstract
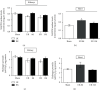
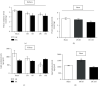
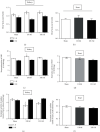
In kidney disease (KD), several factors released into the bloodstream can induce a series of changes in the heart, leading to a wide variety of clinical situations called cardiorenal syndrome (CRS). Reactive oxygen species (ROS) play an important role in the signaling and progression of systemic inflammatory conditions, as observed in KD. The aim of the present study was to characterize the redox balance in renal ischemia/reperfusion-induced cardiac remodeling. C57BL/6 male mice were subjected to occlusion of the left renal pedicle, unilateral, for 60 min, followed by reperfusion for 8 and 15 days, respectively. The following redox balance components were evaluated: catalase (CAT), superoxide dismutase (SOD), total antioxidant capacity (FRAP), NADPH oxidase (NOX), nitric oxide synthase (NOS), hydrogen peroxide (H2O2), and the tissue bioavailability of nitric oxide (NO) such as S-nitrosothiol (RSNO) and nitrite (NO2 -). The results indicated a process of renoprotection in both kidneys, indicated by the reduction of cellular damage and some oxidant agents. We also observed an increase in the activity of antioxidant enzymes, such as SOD, and an increase in NO bioavailability. In the heart, we noticed an increase in the activity of NOX and NOS, together with increased cell damage on day 8, followed by a reduction in protein damage on day 15. The present study concludes that the kidneys and heart undergo distinct processes of damage and repair at the analyzed times, since the heart is a secondary target of ischemic kidney injury. These results are important for a better understanding of the cellular mechanisms involved in CRS.
Copyright © 2020 Wellington Caio-Silva et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Li Q., Wei G., Tao T. Leukocyte immunoglobulin-like receptor B4 (LILRB4) negatively mediates the pathological cardiac hypertrophy by suppressing fibrosis, inflammation and apoptosis via the activation of NF-κB signaling. Biochemical and Biophysical Research Communications. 2019;509(1):16–23. doi: 10.1016/j.bbrc.2018.11.137. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

