Association Use of Bisphosphonates with Risk of Breast Cancer: A Meta-Analysis
- PMID: 33102580
- PMCID: PMC7568169
- DOI: 10.1155/2020/5606573
Association Use of Bisphosphonates with Risk of Breast Cancer: A Meta-Analysis
Abstract
Background: Previous studies have investigated the association between the use of bisphosphonates and the development of breast cancer, which presented controversial results. Thus, this meta-analysis was conducted to summarize the current evidence of the association of bisphosphonate use with breast cancer risk.
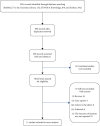
Methods: A comprehensive search was conducted in PubMed, ISI Web of Knowledge, the Cochrane Library, and Embase from inception to March 2019 by two researches, who independently selected trials, retrieved relevant data, and assessed study quality. The summary relative risk (RR) for the use of bisphosphonates on the risks of developing breast cancer was calculated using a random-effect model.
Results: The present meta-analysis, which included four case-control studies, involving 55052 breast cancer cases, and seven retrospective cohort studies, involving 14641 breast cancer cases, assessed the effect of bisphosphonates on breast cancer risk. The random-effect model meta-analysis found a reduced risk of breast cancer with exposure to bisphosphonates with pooled RR of 0.87 (95% confidence interval [CI]: 0.80 to 0.94). The short-term use of bisphosphonates (<1 year) did not render significant alteration (RR = 0.92, 95% CI: 0.82 to 1.03), while a significant 26% risk reduction of breast cancer was noted with long-term use (>1 year) (RR = 0.74, 95% CI: 0.62 to 0.90). A protective effect of bisphosphonates was shown in contralateral breast cancer (RR = 0.41, 95% CI: 0.20 to 0.84). In terms of the type of bisphosphonates, a significant inverse relationship was noted for etidronate, with pooled RR of 0.87 (95% CI: 0.80 to 0.96).
Conclusion: This meta-analysis suggested that the use of bisphosphonates was associated with reduced risk of breast cancer, including contralateral breast cancer. Compared to other types of bisphosphonates, only etidronate showed a significant inverse relationship. Additionally, the long-term use (>1 year) of bisphosphonates was more significant in lowering breast cancer risk. Further randomized controlled trials are needed to verify this association. This trial is registered with PROSPERO (registration number: CRD42018105024) (registered on 29 August 2018).
Copyright © 2020 Rui Peng et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

