Ephrin-A5 Is Involved in Retinal Neovascularization in a Mouse Model of Oxygen-Induced Retinopathy
- PMID: 33102589
- PMCID: PMC7569469
- DOI: 10.1155/2020/7161027
Ephrin-A5 Is Involved in Retinal Neovascularization in a Mouse Model of Oxygen-Induced Retinopathy
Abstract
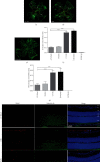
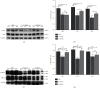
Retinal neovascularization (RNV) is an important pathological feature of vitreoretinopathy that can lead to severe vision loss. The purpose of this study was to identify the role of ephrin-A5 (Efna5) in RNV and to explore its mechanism. The expression pattern and biological significance of Efna5 were investigated in a mouse model of oxygen-induced retinopathy (OIR). The expression of Efna5 and downstream signaling pathway members was determined by RT-PCR, immunofluorescence, immunohistochemistry, and western blot analyses. shRNA was used to knockdown Efna5 in the retina of the OIR mouse model. Retinal flat mounts were performed to evaluate the impact of Efna5 silencing on the RNV process. We found that the Efna5 was greatly upregulated in the retina of OIR mice. Elevated Efna5 mainly colocalized with the retinal vessels and endothelial cells. We then showed that knockdown of Efna5 in OIR mouse retinas using lentivirus-mediated shRNA markedly decreased the expression of Efna5 and reduced the retinal neovascularization and avascular retina area. We further showed hypoxia stimulation dramatically increased both total and phosphorylation levels of ERK1/2 and the phosphorylation levels of Akt in OIR mice. More importantly, knockdown of Efna5 could inhibit the p-Akt and p-ERK signaling pathways. Our results suggested that Efna5 may regulate the RNV. This study suggests that Efna5 was significantly upregulated in the retina of OIR mice and closely involved in the pathological retinal angiogenesis.
Copyright © 2020 Wei Du et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

