The metabolic basis of nonalcoholic steatohepatitis
- PMID: 33102794
- PMCID: PMC7576253
- DOI: 10.1002/edm2.112
The metabolic basis of nonalcoholic steatohepatitis
Abstract
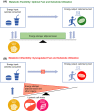
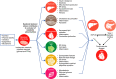
Nonalcoholic fatty liver disease (NAFLD) is a major cause of chronic liver disease and is associated with significant morbidity and mortality worldwide, with a high incidence in Western countries and non-Western countries that have adopted a Western diet. NAFLD is commonly associated with components of the metabolic syndrome, type 2 diabetes mellitus and cardiovascular disease, suggesting a common mechanistic basis. An inability to metabolically handle free fatty acid overload-metabolic inflexibility-constitutes a core node for NAFLD pathogenesis, with resulting lipotoxicity, mitochondrial dysfunction and cellular stress leading to inflammation, apoptosis and fibrogenesis. These responses can lead to the histological phenotype of nonalcoholic steatohepatitis (NASH) with varying degrees of fibrosis, which can progress to cirrhosis. This perspective review describes the key cellular and molecular mechanisms of NAFLD and NASH, namely an excessive burden of carbohydrates and fatty acids that contribute to lipotoxicity resulting in hepatocellular injury and fibrogenesis. Understanding the extrahepatic dysmetabolic contributors to NASH is crucial for the development of safe, effective and durable treatment approaches for this increasingly common disease.
Keywords: insulin resistance; lipotoxic stress; metabolic inflexibility; mitochondrial dysfunction.
© 2020 The Authors. Endocrinology, Diabetes & Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
MVC is an employee of Axcella Health, Inc and owns stock options in the company. BAN‐T is a consultant for Allergan, Allysta, Arrowhead, ARTham, Axcella, Blade, Boehringer Ingelheim, BMS, Coherus, Consynance, Durect, Enanta, Fortress, Gelesis, Gilead, HistoIndex, Intercept, Lipocine, Madrigal, Medimmune, Merck, Metacrine, Mundipharma, NGM, pH‐Pharma, Prometheus and Siemens. He has received institutional research grants from Allergan, BMS, Cirius, Cymabay, Enanta, Galectin, Genfit, Gilead, Intercept, Madrigal, NGM and Prometheus.
Figures



References
-
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73‐84. - PubMed
-
- Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388‐1393. - PubMed
-
- Konerman MA, Jones JC, Harrison SA. Pharmacotherapy for NASH: current and emerging. J Hepatol. 2018;68(2):362‐375. - PubMed
-
- Ahmed A, Wong RJ, Harrison SA. Nonalcoholic fatty liver disease review: diagnosis, treatment, and outcomes. Clin Gastroenterol Hepatol. 2015;13(12):2062‐2070. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

