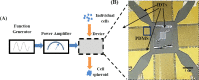
Formation of cell spheroids using Standing Surface Acoustic Wave (SSAW)
- PMID: 33102912
- PMCID: PMC7582004
- DOI: 10.18063/IJB.v4i1.130
Formation of cell spheroids using Standing Surface Acoustic Wave (SSAW)
Abstract
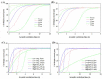
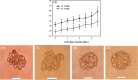
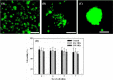
3D bioprinting becomes one of the popular approaches in the tissue engineering. In this emerging application, bioink is crucial for fabrication and functionality of constructed tissue. The use of cell spheroids as bioink can enhance the cell-cell interaction and subsequently the growth and differentiation of cells in the 3D printed construct with the minimum amount of other biomaterials. However, the conventional methods of preparing the cell spheroids have several limitations, such as long culture time, low-throughput, and medium modification. In this study, the formation of cell spheroids by SSAW was evaluated both numerically and experimentally in order to overcome the aforementioned limitations. The effects of excitation frequencies on the cell accumulation time, diameter of the formed cell spheroids, and subsequently, the growth and viability of cell spheroids in the culture medium over time were studied. Using the high-frequency (23.8 MHz) excitation, cell accumulation time to the pressure nodes could be reduced in comparison to that of the low-frequency (10.4 MHz) excitation, but in a smaller spheroid size. SSAW excitation at both frequencies does not affect the cell viability up to 7 days, > 90% with no statistical difference compared with the control group. In summary, SSAW can effectively prepare the cell spheroids as bioink for the future 3D bioprinting and various biotechnology applications (e.g., pharmaceutical drug screening and tissue engineering).
Keywords: bioink; cell spheroid; cell viability; interdigital transducer (IDT); standing surface acoustic wave (SSAW).
Copyright: © 2018 Sriphutkiat Y, et al.
Conflict of interest statement
No conflict of interest was reported by all the authors.
Figures

Similar articles
-
A Simple and Efficient Strategy for Preparing a Cell-Spheroid-Based Bioink.Adv Healthc Mater. 2022 Aug;11(15):e2200648. doi: 10.1002/adhm.202200648. Epub 2022 Jun 17. Adv Healthc Mater. 2022. PMID: 35543489
-
Engineered biomaterials to guide spheroid formation, function, and fabrication into 3D tissue constructs.Acta Biomater. 2023 Jul 15;165:4-18. doi: 10.1016/j.actbio.2022.09.052. Epub 2022 Sep 24. Acta Biomater. 2023. PMID: 36167240 Free PMC article. Review.
-
The Application of Ultrasound in 3D Bio-Printing.Molecules. 2016 May 5;21(5):590. doi: 10.3390/molecules21050590. Molecules. 2016. PMID: 27164066 Free PMC article. Review.
-
High-throughput fabrication of cell spheroids with 3D acoustic assembly devices.Int J Bioprint. 2023 Apr 17;9(4):733. doi: 10.18063/ijb.733. eCollection 2023. Int J Bioprint. 2023. PMID: 37323490 Free PMC article.
-
Scaffold-free bioprinting of mesenchymal stem cells using the Regenova printer: Spheroid characterization and osteogenic differentiation.Bioprinting. 2019 Sep;15:e00050. doi: 10.1016/j.bprint.2019.e00050. Epub 2019 Apr 23. Bioprinting. 2019. PMID: 31457109 Free PMC article.
Cited by
-
Ultrasound-Based Scaffold-Free Core-Shell Multicellular Tumor Spheroid Formation.Micromachines (Basel). 2021 Mar 20;12(3):329. doi: 10.3390/mi12030329. Micromachines (Basel). 2021. PMID: 33804708 Free PMC article.
-
Acoustic Manipulation of Intraocular Particles.Micromachines (Basel). 2022 Aug 21;13(8):1362. doi: 10.3390/mi13081362. Micromachines (Basel). 2022. PMID: 36014284 Free PMC article.
-
Uncovering 3D bioprinting research trends: A keyword network mapping analysis.Int J Bioprint. 2018 Jul 9;4(2):147. doi: 10.18063/IJB.v4i2.147. eCollection 2018. Int J Bioprint. 2018. PMID: 33102921 Free PMC article.
-
The waves that make the pattern: a review on acoustic manipulation in biomedical research.Mater Today Bio. 2021 Mar 24;10:100110. doi: 10.1016/j.mtbio.2021.100110. eCollection 2021 Mar. Mater Today Bio. 2021. PMID: 33997761 Free PMC article. Review.
-
Discovering the Latest Scientific Pathways on Tissue Spheroids: Opportunities to Innovate.Int J Bioprint. 2021 Jan 29;7(1):331. doi: 10.18063/ijb.v7i1.331. eCollection 2021. Int J Bioprint. 2021. PMID: 33585717 Free PMC article.
References
-
- Ng W L, Wang S, Yeong W Y, et al. Skin bioprinting:Impending reality or fantasy? Trends Biotechnol. 2016;35(3):278. http://dx.doi.org/10.1016/j.tibtech.2016.04.006. - PubMed
-
- Ng W L, Tan J, Yeong W Y, et al. Proof-ofconcept:3D bioprinting of pigmented human skin constructs. Biofabrication. 2018:10. - PubMed
-
- Suntornnond R, Tan E Y S, An J, et al. A highly printable and biocompatible hydrogel composite for direct printing of soft and perfusable vasculature-like structures. Sci Rep. 2017;7(1):16902. http://dx.doi.org/10.1038/s41598-017-17198-0. - PMC - PubMed
-
- Olubamiji A D, Izadifar Z, Si J L, et al. Modulating mechanical behaviour of 3D-printed cartilage -mimetic PCL scaffolds:Influence of molecular weight and pore geometry. Biofabrication. 2016;8(2):025020. http://dx.doi.org/10.1088/1758-5090/8/2/025020. - PubMed
-
- Sing S L, An J, Yeong W Y, et al. Laser and electron-beam powder-bed additive manufacturing of metallic implants:A review on processes, materials and designs. J Orthop Res. 2016;34(3):369–385. http://dx.doi.org/10.1002/jor.23075. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
