The arrival of commercial bioprinters - Towards 3D bioprinting revolution!
- PMID: 33102917
- PMCID: PMC7582003
- DOI: 10.18063/IJB.v4i2.139
The arrival of commercial bioprinters - Towards 3D bioprinting revolution!
Abstract
The dawn of commercial bioprinting is rapidly advancing the tissue engineering field. In the past few years, new bioprinting approaches as well as novel bioinks formulations have emerged, enabling biological research groups to demonstrate the use of such technology to fabricate functional and relevant tissue models. In recent years, several companies have launched bioprinters pushing for early adoption and democratisation of bioprinting. This article reviews the progress in commercial bioprinting since the inception, with a particular focus on the comparison of different available printing technologies and important features of the individual technologies as well as various existing applications. Various challenges and potential design considerations for next generations of bioprinters are also discussed.
Keywords: 3D printing; Bioprinter; biofabrication; bioinks; bioprinting; rapid prototyping; tissue engineering.
Copyright: © 2018 Choudhury D, et al.
Conflict of interest statement
All authors reported no conflict of interest.
Figures
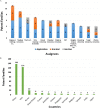





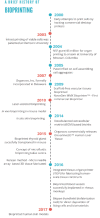

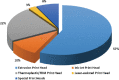
References
-
- Ozbolat IT. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 2015;33(7):395–400. http://dx.doi.org/10.1016/j.tibtech.2015.04.005. - PubMed
-
- Murphy S, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. http://dx.doi.org/10.1038/nbt.2958. - PubMed
-
- Gungor-Ozkerim P S, Inci I, Zhang Y S, et al. Bioinks for 3D bioprinting:An overview. Biomater Sci. 2018;6(5):915946. http://dx.doi.org/10.1039/C7BM00765E. - PMC - PubMed
-
- Mironov V, Boland T, Trusk T, et al. Organ printing:Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21(4):157–161. https://dx.doi.org/10.1016/ S0167-7799(03)00033-7. - PubMed
-
- Holzl K, Lin S, Tytgat L, et al. Bioink properties before, during and after 3D bioprinting. Biofabrication. 2016;8(3):032002. https://doi.org/10.1088/1758-5090/8/3/032002. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
