Gadoxetic acid-based hepatobiliary MRI in hepatocellular carcinoma
- PMID: 33103093
- PMCID: PMC7578758
- DOI: 10.1016/j.jhepr.2020.100173
Gadoxetic acid-based hepatobiliary MRI in hepatocellular carcinoma
Abstract
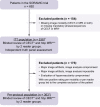
Background & aims: SORAMIC is a prospective phase II randomised controlled trial in hepatocellular carcinoma (HCC). It consists of 3 parts: a diagnostic study and 2 therapeutic studies with either curative ablation or palliative Yttrium-90 radioembolisation combined with sorafenib. We report the diagnostic cohort study aimed to determine the accuracy of gadoxetic acid-enhanced magnetic resonance imaging (MRI), including hepatobiliary phase (HBP) imaging features compared with contrast-enhanced computed tomography (CT). The primary objective was the accuracy of treatment decisions stratifying patients for curative or palliative (non-ablation) treatment.
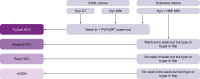
Methods: Patients with clinically suspected HCC underwent gadoxetic acid-enhanced MRI (HBP MRI, including dynamic MRI) and contrast-enhanced CT. Blinded read of the image data was performed by 2 reader groups (radiologists, R1 and R2). A truth panel with access to all clinical data and follow-up imaging served as reference. Imaging criteria for curative ablation were defined as up to 4 lesions <5 cm and absence of macrovascular invasion. The primary endpoint was non-inferiority of HBP MRI vs. CT in a first step and superiority in a second step.
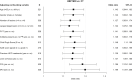
Results: The intent-to-treat population comprised 538 patients. Treatment decisions matched the truth panel assessment in 83.3% and 81.2% for HBP MRI (R1 and R2), and 73.4% and 70.8% for CT. Non-inferiority and superiority (second step) of HBP MRI vs. CT were demonstrated (odds ratio 1.14 [1.09-1.19]). HBP MRI identified patients with >4 lesions significantly more frequently than CT.
Conclusions: In HCC, HBP MRI provided a more accurate decision than CT for a curative vs. palliative treatment strategy.
Lay summary: Patients with hepatocellular carcinoma are allocated to curative or palliative treatment according to the stage of their disease. Hepatobiliary imaging using gadoxetic acid-enhanced MRI is more accurate than CT for treatment decision-making.
Keywords: APASL, Asian Pacific Association for the Study of the Liver; BCLC, Barcelona Clinic Liver Cancer; CT, computed tomography; DWI, diffusion-weighted imaging; GEE, generalised estimating equation; GRE, gradient echo; Gadoxetic acid; HBP, hepatobiliary phase; HCC, hepatocellular carcinoma; HGDN, high-grade dysplastic nodule; Hepatocellular carcinoma; ITT, intent to treat; MRI, magnetic resonance imaging; Magnetic resonance imaging; OR, odds ratio; PP, per protocol; RFA, radio-frequency ablation; SORAMIC trial; SORAMIC, Sorafenib and Micro-Therapy Guided by Gadolinium-EOB-DTPA-Enhanced MRI; TSE, turbo spin echo.
© 2020 The Author(s).
Conflict of interest statement
Jens Ricke reports grants and personal fees from Sirtex Medical and Bayer HealthCare. Ingo G. Steffen reports grants from Sirtex Medical and Bayer HealthCare. Irene Bargellini reports grants and personal fees from BTG Ltd, Sirtex Medical, Terumo Europe, GE Healthcare, and Bayer HealthCare. Thomas Berg currently acts as an advisor to AbbVie, Alexion, Bayer, Bristol Myers Squibb, Gilead, Intercept, Janssen, MSD/Merck, Merz, Novartis, and Sequana Medical. He has received speaking honoraria from AbbVie, Alexion, Bayer, Bristol Myers Squibb, Eisai, Gilead, Intercept, Ipsen, Janssen, MSD/Merck, Merz, Novartis, Sirtex, and Sequana Medical in the past 2 yr. He has received grant support from AbbVie, Bristol Myers Squibb, Gilead, Humedics, Intercept, Janssen, MSD/Merck, Merz, Novartis, and Sequana Medical. José Ignacio Bilbao Jaureguizar reports grants and personal fees from Sirtex Medical. Bernhard Gebauer reports personal fees and non-financial support from Sirtex Medical; personal fees and non-financial support from Bayer HealthCare; personal fees from Sirtex Medical, Pfizer, Merck, ICON, Parexel, BD/CR BARD, Roche, Guerbet, and Eisai. Roberto Iezzi reports grants and personal fees from Sirtex Medical. Musturay Karçaaltincaba reports personal fees from Bayer, GE Healthcare, and Pfizer. Maciej Pech reports grants from Bayer HealthCare, and grants and personal fees from Sirtex. Christoph J. Zech reports grants and personal fees from Bayer HealthCare. Max Seidensticker reports grants and personal fees from Sirtex and Bayer HealthCare. The other authors report nothing to disclose. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- Perz J.F., Armstrong G.L., Farrington L.A., Hutin Y.J., Bell B.P. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. - PubMed
-
- Mazzaferro V., Llovet J.M., Miceli R., Bhoori S., Schiavo M., Mariani L. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. - PubMed
-
- Vogel A., Cervantes A., Chau I., Daniele B., Llovet J.M., Meyer T. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl. 4):iv238–v255. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

