Reaction mechanism of tetrathionate hydrolysis based on the crystal structure of tetrathionate hydrolase from Acidithiobacillus ferrooxidans
- PMID: 33103311
- PMCID: PMC7784748
- DOI: 10.1002/pro.3984
Reaction mechanism of tetrathionate hydrolysis based on the crystal structure of tetrathionate hydrolase from Acidithiobacillus ferrooxidans
Abstract
Tetrathionate hydrolase (4THase) plays an important role in dissimilatory sulfur oxidation in the acidophilic iron- and sulfur-oxidizing bacterium Acidithiobacillus ferrooxidans. The structure of recombinant 4THase from A. ferrooxidans (Af-Tth) was determined by X-ray crystallography to a resolution of 1.95 Å. Af-Tth is a homodimer, and its monomer structure exhibits an eight-bladed β-propeller motif. Two insertion loops participate in dimerization, and one loop forms a cavity with the β-propeller region. We observed unexplained electron densities in this cavity of the substrate-soaked structure. The anomalous difference map generated using diffraction data collected at a wavelength of 1.9 Å indicated the presence of polymerized sulfur atoms. Asp325, a highly conserved residue among 4THases, was located near the polymerized sulfur atoms. 4THase activity was completely abolished in the site-specific Af-Tth D325N variant, suggesting that Asp325 plays a crucial role in the first step of tetrathionate hydrolysis. Considering that the Af-Tth reaction occurs only under acidic pH, Asp325 acts as an acid for the tetrathionate hydrolysis reaction. The polymerized sulfur atoms in the active site cavity may represent the intermediate product in the subsequent step.
Keywords: Acidithiobacillus ferrooxidans; hydrolase; protein tertiary structure; site-directed mutagenesis; sulfur oxidation; tetrathionic acid.
© 2020 The Protein Society.
Conflict of interest statement
All authors declare that they have no conflicts of interest with respect to the publication of this article.
Figures
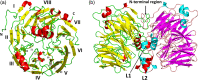




Similar articles
-
Tetrathionate hydrolase from the acidophilic microorganisms.Front Microbiol. 2024 Jan 29;15:1338669. doi: 10.3389/fmicb.2024.1338669. eCollection 2024. Front Microbiol. 2024. PMID: 38348185 Free PMC article. Review.
-
Identification of a gene encoding a tetrathionate hydrolase in Acidithiobacillus ferrooxidans.J Biotechnol. 2007 Oct 15;132(1):16-22. doi: 10.1016/j.jbiotec.2007.08.030. Epub 2007 Aug 21. J Biotechnol. 2007. PMID: 17904676
-
The sole cysteine residue (Cys301) of tetrathionate hydrolase from Acidithiobacillus ferrooxidans does not play a role in enzyme activity.Biosci Biotechnol Biochem. 2014;78(12):2030-5. doi: 10.1080/09168451.2014.948374. Epub 2014 Aug 21. Biosci Biotechnol Biochem. 2014. PMID: 25144400
-
Crystallization and preliminary X-ray diffraction analysis of tetrathionate hydrolase from Acidithiobacillus ferrooxidans.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013 Jun;69(Pt 6):692-4. doi: 10.1107/S1744309113013419. Epub 2013 May 29. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013. PMID: 23722856 Free PMC article.
-
Iron and sulfur oxidation pathways of Acidithiobacillus ferrooxidans.World J Microbiol Biotechnol. 2019 Mar 27;35(4):60. doi: 10.1007/s11274-019-2632-y. World J Microbiol Biotechnol. 2019. PMID: 30919119 Review.
Cited by
-
AI-driven pan-proteome analyses reveal insights into the biohydrometallurgical properties of Acidithiobacillia.Front Microbiol. 2023 Sep 7;14:1243987. doi: 10.3389/fmicb.2023.1243987. eCollection 2023. Front Microbiol. 2023. PMID: 37744906 Free PMC article.
-
Overlooked role of heterotrophic prokaryotes in sulfur oxidation makes the sediment of the Bohai Sea a sufficient sink of hydrogen sulfide.mBio. 2025 Aug 13;16(8):e0172225. doi: 10.1128/mbio.01722-25. Epub 2025 Jul 7. mBio. 2025. PMID: 40622149 Free PMC article.
-
Characterization of Tetrathionate Hydrolase from Acidothermophilic Sulfur-Oxidizing Archaeon Metallosphaera cuprina Ar-4.Int J Mol Sci. 2025 Feb 5;26(3):1338. doi: 10.3390/ijms26031338. Int J Mol Sci. 2025. PMID: 39941105 Free PMC article.
-
Isolation, Sphalerite Bioleaching, and Whole Genome Sequencing of Acidithiobacillus ferriphilus QBS3 from Zinc-Rich Sulfide Mine Drainage.Life (Basel). 2025 May 15;15(5):792. doi: 10.3390/life15050792. Life (Basel). 2025. PMID: 40430218 Free PMC article.
-
Tetrathionate hydrolase from the acidophilic microorganisms.Front Microbiol. 2024 Jan 29;15:1338669. doi: 10.3389/fmicb.2024.1338669. eCollection 2024. Front Microbiol. 2024. PMID: 38348185 Free PMC article. Review.
References
-
- Bugaytsova Z, Lindström EB. Localization, purification and properties of a tetrathionate hydrolase from Acidithiobacillus caldus . Eur J Biochem. 2004;271:272–280. - PubMed
-
- Tano T, Kitaguchi H, Harada M, Nagasawa T, Sugio T. Purification and some properties of a tetrathionate decomposing enzyme from Thiobacillus thiooxidans . Biosci Biotechnol Biochem. 1996;60:224–227. - PubMed
-
- Kanao T, Onishi M, Kajitani Y, et al. Characterization of tetrathionate hydrolase from the marine acidophilic sulfur‐oxidizing bacterium, Acidithiobacillus thiooxidans strain SH. Biosci Biotechnol Biochem. 2018;82:152–160. - PubMed
-
- Kanao T, Kamimura K, Sugio T. Identification of a gene encoding a tetrathionate hydrolase in Acidithiobacillus ferrooxidans . J Biotechnol. 2007;132:16–22. - PubMed
-
- de Jong GAH, Hazeu W, Bos P, Kuenen JG. Isolation of the tetrathionate hydrolase from Thiobacillus acidophilus . Eur J Biochem. 1997;243:678–683. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

