Total Synthesis of the Diterpene Waihoensene
- PMID: 33103334
- PMCID: PMC7898921
- DOI: 10.1002/anie.202011298
Total Synthesis of the Diterpene Waihoensene
Abstract
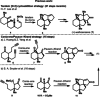
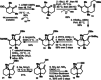
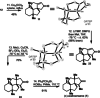
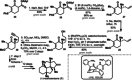
A racemic and scalable enantioselective total synthesis of (+)-waihoensene was accomplished. (+)-Waihoensene belongs to the diterpene natural product family, and it features an angular triquinane substructure motif. Its tetracyclic [6.5.5.5]backbone is all-cis-fused, containing six contiguous stereocenters, four of which are quaternary. These structural features were efficiently installed by means of a diastereoselective radical cyclization, followed by an intramolecular Pauson-Khand reaction, a diastereoselective α-alkylation, and a diastereoselective 1,4-addition reaction. Enantioselectivity was introduced at an early stage, by an asymmetric palladium catalyzed decarboxylative allylation reaction on gram scale.
Keywords: Pauson-Khand reaction; natural products; radicals; terpenes; total synthesis.
© 2020 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Asymmetric Total Synthesis of (+)-Waihoensene.J Am Chem Soc. 2020 Apr 8;142(14):6511-6515. doi: 10.1021/jacs.0c02143. Epub 2020 Mar 26. J Am Chem Soc. 2020. PMID: 32203659
-
A Concise Total Synthesis of (+)-Waihoensene Guided by Quaternary Center Analysis.Angew Chem Int Ed Engl. 2020 Aug 3;59(32):13521-13525. doi: 10.1002/anie.202004177. Epub 2020 Jun 3. Angew Chem Int Ed Engl. 2020. PMID: 32330370 Free PMC article.
-
Total Synthesis of (±)-Waihoensene.Angew Chem Int Ed Engl. 2017 Jul 3;56(28):8254-8257. doi: 10.1002/anie.201704492. Epub 2017 Jun 13. Angew Chem Int Ed Engl. 2017. PMID: 28544050
-
Enantioselective formation of quaternary carbon stereocenters in natural product synthesis: a recent update.Nat Prod Rep. 2020 Feb 26;37(2):276-292. doi: 10.1039/c9np00039a. Nat Prod Rep. 2020. PMID: 31515549 Review.
-
Enantioselective total syntheses of several bioactive natural products based on the development of practical asymmetric catalysis.Chem Pharm Bull (Tokyo). 2004 Sep;52(9):1031-52. doi: 10.1248/cpb.52.1031. Chem Pharm Bull (Tokyo). 2004. PMID: 15340187 Review.
Cited by
-
Total Synthesis of Terpenes and Their Biological Significance: A Critical Review.Pharmaceuticals (Basel). 2022 Nov 11;15(11):1392. doi: 10.3390/ph15111392. Pharmaceuticals (Basel). 2022. PMID: 36422521 Free PMC article. Review.
-
Total syntheses of strained polycyclic terpenes.Chem Commun (Camb). 2022 Apr 19;58(32):4941-4953. doi: 10.1039/d2cc00926a. Chem Commun (Camb). 2022. PMID: 35388836 Free PMC article. Review.
-
Stereo-Differentiating Asymmetric Rh(I)-Catalyzed Pauson-Khand Reaction: A DFT-Informed Approach to Thapsigargin Stereoisomers.J Am Chem Soc. 2025 Jan 8;147(1):498-509. doi: 10.1021/jacs.4c11661. Epub 2024 Dec 19. J Am Chem Soc. 2025. PMID: 39702925 Free PMC article.
-
Finding activity through rigidity: syntheses of natural products containing tricyclic bridgehead carbon centers.Nat Prod Rep. 2023 Aug 16;40(8):1393-1431. doi: 10.1039/d3np00008g. Nat Prod Rep. 2023. PMID: 37140079 Free PMC article. Review.
-
Synthetic study toward the diterpenoid aberrarone.Beilstein J Org Chem. 2022 Nov 30;18:1625-1628. doi: 10.3762/bjoc.18.173. eCollection 2022. Beilstein J Org Chem. 2022. PMID: 36530530 Free PMC article.
References
-
- None
-
- Mehta G., Srikrishna G., Chem. Rev. 1997, 97, 671–720; - PubMed
-
- Clarke D. B., Hinkley S. F. R., Weavers R. T., Tetrahedron Lett. 1997, 38, 4297–4300.
LinkOut - more resources
Full Text Sources

