Histone deacetylase 6 inhibition mitigates renal fibrosis by suppressing TGF-β and EGFR signaling pathways in obstructive nephropathy
- PMID: 33103445
- PMCID: PMC7792693
- DOI: 10.1152/ajprenal.00261.2020
Histone deacetylase 6 inhibition mitigates renal fibrosis by suppressing TGF-β and EGFR signaling pathways in obstructive nephropathy
Abstract
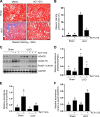
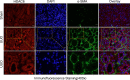
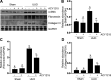
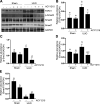
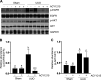
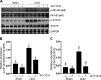
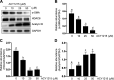
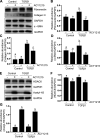
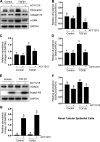
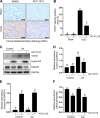
We have recently shown that histone deacetylase 6 (HDAC6) is critically involved in the pathogenesis of acute kidney injury. Its role in renal fibrosis, however, remains unclear. In this study, we examined the effect of ricolinostat (ACY-1215), a selective inhibitor of HDAC6, on the development of renal fibrosis in a murine model induced by unilateral ureteral obstruction (UUO). HDAC6 was highly expressed in the kidney following UUO injury, which was coincident with deposition of collagen fibrils and expression of α-smooth muscle actin, fibronectin, and collagen type III. Administration of ACY-1215 reduced these fibrotic changes and inhibited UUO-induced expression of transforming growth factor-β1 and phosphorylation of Smad3 while increasing expression of Smad7. ACY-1215 treatment also suppressed phosphorylation of epidermal growth factor receptor (EGFR) and several signaling molecules associated with renal fibrogenesis, including AKT, STAT3, and NF-κB in the injured kidney. Furthermore, ACY-1215 was effective in inhibiting dedifferentiation of renal fibroblasts to myofibroblasts and the fibrotic change of renal tubular epithelial cells in culture. Collectively, these results indicate that HDAC6 inhibition can attenuate development of renal fibrosis by suppression of transforming growth factor-β1 and EGFR signaling and suggest that HDAC6 would be a potential therapeutic target for the treatment of renal fibrosis.
Keywords: ACY-1215; epidermal growth factor receptor; histone deacetylase 6; renal fibrosis; transforming growth factor-β1; unilateral ureteral obstruction.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
HDAC6 inhibition: a significant potential regulator and therapeutic option to translate into clinical practice in renal transplantation.Front Immunol. 2023 Jul 21;14:1168848. doi: 10.3389/fimmu.2023.1168848. eCollection 2023. Front Immunol. 2023. PMID: 37545520 Free PMC article. Review.
-
Blocking the class I histone deacetylase ameliorates renal fibrosis and inhibits renal fibroblast activation via modulating TGF-beta and EGFR signaling.PLoS One. 2013;8(1):e54001. doi: 10.1371/journal.pone.0054001. Epub 2013 Jan 16. PLoS One. 2013. PMID: 23342059 Free PMC article.
-
Apamin inhibits renal fibrosis via suppressing TGF-β1 and STAT3 signaling in vivo and in vitro.J Mol Med (Berl). 2021 Sep;99(9):1265-1277. doi: 10.1007/s00109-021-02087-x. Epub 2021 May 24. J Mol Med (Berl). 2021. PMID: 34031696
-
Petchiether A attenuates obstructive nephropathy by suppressing TGF-β/Smad3 and NF-κB signalling.J Cell Mol Med. 2019 Aug;23(8):5576-5587. doi: 10.1111/jcmm.14454. Epub 2019 Jun 18. J Cell Mol Med. 2019. PMID: 31211499 Free PMC article.
-
Pro- and anti-fibrotic effects of vascular endothelial growth factor in chronic kidney diseases.Ren Fail. 2022 Dec;44(1):881-892. doi: 10.1080/0886022X.2022.2079528. Ren Fail. 2022. PMID: 35618410 Free PMC article. Review.
Cited by
-
NSC828779 Alleviates Renal Tubulointerstitial Lesions Involving Interleukin-36 Signaling in Mice.Cells. 2021 Nov 6;10(11):3060. doi: 10.3390/cells10113060. Cells. 2021. PMID: 34831283 Free PMC article.
-
Roles of Epigenetics in Cardiac Fibroblast Activation and Fibrosis.Cells. 2022 Jul 30;11(15):2347. doi: 10.3390/cells11152347. Cells. 2022. PMID: 35954191 Free PMC article. Review.
-
HDAC11 promotes renal fibrosis by induing partial epithelial-mesenchymal transition and G2/M phase arrest in renal epithelial cells.Res Sq [Preprint]. 2025 May 6:rs.3.rs-6523050. doi: 10.21203/rs.3.rs-6523050/v1. Res Sq. 2025. PMID: 40386380 Free PMC article. Preprint.
-
HDAC6 inhibition: a significant potential regulator and therapeutic option to translate into clinical practice in renal transplantation.Front Immunol. 2023 Jul 21;14:1168848. doi: 10.3389/fimmu.2023.1168848. eCollection 2023. Front Immunol. 2023. PMID: 37545520 Free PMC article. Review.
-
The role of HDAC6 in fibrosis: a novel and effective therapy strategy.Eur J Med Res. 2025 Jul 4;30(1):571. doi: 10.1186/s40001-025-02840-9. Eur J Med Res. 2025. PMID: 40615924 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

