Pharmacophore-based virtual screening, synthesis, biological evaluation, and molecular docking study of novel pyrrolizines bearing urea/thiourea moieties with potential cytotoxicity and CDK inhibitory activities
- PMID: 33103497
- PMCID: PMC7594867
- DOI: 10.1080/14756366.2020.1837124
Pharmacophore-based virtual screening, synthesis, biological evaluation, and molecular docking study of novel pyrrolizines bearing urea/thiourea moieties with potential cytotoxicity and CDK inhibitory activities
Abstract
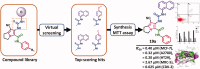
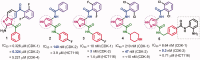
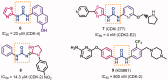
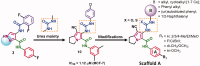
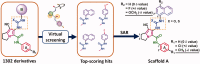
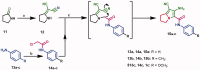
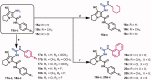
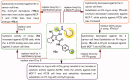
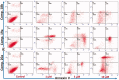
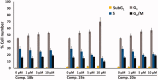
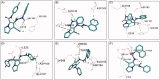
In the current study, virtual screening of a small library of 1302 pyrrolizines bearing urea/thiourea moieties was performed. The top-scoring hits were synthesised and evaluated for their cytotoxicity against three cancer (MCF-7, A2780, and HT29) and one normal (MRC-5) cell lines. The results of the MTT assay revealed potent cytotoxic activities for most of the new compounds (IC50 = 0.16-34.13 μM). The drug-likeness study revealed that all the new compounds conform to Lipinski's rule. Mechanistic studies of compounds 18 b, 19a, and 20a revealed the induction of apoptosis and cell cycle arrest at the G1 phase in MCF-7 cells. The three compounds also displayed potent inhibitory activity against CDK-2 (IC50 = 25.53-115.30 nM). Moreover, the docking study revealed a nice fitting of compound 19a into the active sites of CDK-2/6/9. These preliminary results suggested that compound 19a could serve as a promising scaffold in the discovery of new potent anticancer agents.
Keywords: CDK-2; Pyrrolizine; apoptosis; cell cycle; cytotoxicity; urea derivatives.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


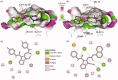
Similar articles
-
Ethyl benzoate bearing pyrrolizine/indolizine moieties: Design, synthesis and biological evaluation of anti-inflammatory and cytotoxic activities.Bioorg Chem. 2020 Jan;94:103371. doi: 10.1016/j.bioorg.2019.103371. Epub 2019 Oct 24. Bioorg Chem. 2020. PMID: 31708230
-
Discovery of 3,6-disubstituted pyridazines as a novel class of anticancer agents targeting cyclin-dependent kinase 2: synthesis, biological evaluation and in silico insights.J Enzyme Inhib Med Chem. 2020 Dec;35(1):1616-1630. doi: 10.1080/14756366.2020.1806259. J Enzyme Inhib Med Chem. 2020. PMID: 32781872 Free PMC article.
-
Discovery of nitroaryl urea derivatives with antiproliferative properties.J Enzyme Inhib Med Chem. 2016 Aug;31(4):608-18. doi: 10.3109/14756366.2015.1057716. Epub 2015 Jun 26. J Enzyme Inhib Med Chem. 2016. PMID: 26114307
-
2-Anilinopyrimidine derivatives: Design, synthesis, in vitro anti-proliferative activity, EGFR and ARO inhibitory activity, cell cycle analysis and molecular docking study.Bioorg Chem. 2020 Jun;99:103798. doi: 10.1016/j.bioorg.2020.103798. Epub 2020 Mar 29. Bioorg Chem. 2020. PMID: 32247112 Review.
-
Pyrrole-containing hybrids as potential anticancer agents: An insight into current developments and structure-activity relationships.Eur J Med Chem. 2024 Jul 5;273:116470. doi: 10.1016/j.ejmech.2024.116470. Epub 2024 May 3. Eur J Med Chem. 2024. PMID: 38762915 Review.
Cited by
-
Characterization of Promising Cytotoxic Metabolites from Tabebuia guayacan Hemsl.: Computational Prediction and In Vitro Testing.Plants (Basel). 2022 Mar 26;11(7):888. doi: 10.3390/plants11070888. Plants (Basel). 2022. PMID: 35406868 Free PMC article.
-
Fine-tuning of the pharmacological potential of novel thiazolium ionic liquids by anion alteration.RSC Adv. 2021 Dec 22;12(1):458-469. doi: 10.1039/d1ra07128a. eCollection 2021 Dec 20. RSC Adv. 2021. PMID: 35424514 Free PMC article.
-
Novel pyrrolizines bearing 3,4,5-trimethoxyphenyl moiety: design, synthesis, molecular docking, and biological evaluation as potential multi-target cytotoxic agents.J Enzyme Inhib Med Chem. 2021 Dec;36(1):1313-1333. doi: 10.1080/14756366.2021.1937618. J Enzyme Inhib Med Chem. 2021. PMID: 34154478 Free PMC article.
-
In Silico Approach Using Free Software to Optimize the Antiproliferative Activity and Predict the Potential Mechanism of Action of Pyrrolizine-Based Schiff Bases.Molecules. 2021 Jun 30;26(13):4002. doi: 10.3390/molecules26134002. Molecules. 2021. PMID: 34209011 Free PMC article.
-
A pharmacophore-guided deep learning approach for bioactive molecular generation.Nat Commun. 2023 Oct 6;14(1):6234. doi: 10.1038/s41467-023-41454-9. Nat Commun. 2023. PMID: 37803000 Free PMC article.
References
-
- Ferguson FM, Gray NS.. Kinase inhibitors: the road ahead. Nat Rev Drug Discov 2018;17:353–77. - PubMed
-
- Wu P, Nielsen TE, Clausen MH.. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci 2015;36:422–39. - PubMed
-
- Roskoski R Jr. Properties of FDA-approved small molecule protein kinase inhibitors: a 2020 update. Pharmacol Res 2020;152:104609. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
