A ketogenic drink improves cognition in mild cognitive impairment: Results of a 6-month RCT
- PMID: 33103819
- PMCID: PMC8048678
- DOI: 10.1002/alz.12206
A ketogenic drink improves cognition in mild cognitive impairment: Results of a 6-month RCT
Abstract
Introduction: Counteracting impaired brain glucose metabolism with ketones may improve cognition in mild cognitive impairment (MCI).
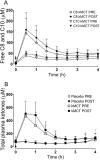
Methods: Cognition, plasma ketone response, and metabolic profile were assessed before and 6 months after supplementation with a ketogenic drink containing medium chain triglyceride (ketogenic medium chain triglyceride [kMCT]; 15 g twice/day; n = 39) or placebo (n = 44).
Results: Free and cued recall (Trial 1; P = .047), verbal fluency (categories; P = .024), Boston Naming Test (total correct answers; P = .033), and the Trail-Making Test (total errors; P = .017) improved significantly in the kMCT group compared to placebo (analysis of covariance; pre-intervention score, sex, age, education, and apolipoprotein E4 as covariates). Some cognitive outcomes also correlated positively with plasma ketones. Plasma metabolic profile and ketone response were unchanged.
Conclusions: This kMCT drink improved cognitive outcomes in MCI, at least in part by increasing blood ketone level. These data support further assessment of MCI progression to Alzheimer's disease.
Keywords: Alzheimer's disease; acetoacetate; beta-hydroxybutyrate; cognition; episodic memory; executive function; ketone; language; medium chain triglyceride; mild cognitive impairment.
© 2020 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Stephen C. Cunnane has consulted for or received travel honoraria or test products for research from Nestlé Health Science, Bulletproof, Cerecin, and Abitec. Stephen C. Cunnane is the founder and director of the consulting company, Senotec Ltd. JPG is an employee of the Société des Produits Nestlé S.A. There are no other conflicts to report.
Figures


References
-
- Morbelli S, Brugnolo A, Bossert I, et al. Visual versus semi‐quantitative analysis of 18F‐FDG‐PET in amnestic MCI: an European Alzheimer's Disease Consortium (EADC) project. J Alzheimers Dis. 2015;44:815‐826. - PubMed
-
- Ishibashi K, Onishi A, Wagatsuma K, Fujiwara Y, Ishii K. Longitudinal 18F‐FDG images in patients with Alzheimer disease over more than 9 years from a preclinical stage. Clin Nucl Med. 2020;45:e185‐e9. - PubMed
-
- Croteau E, Castellano CA, Richard MA, et al. Ketogenic medium chain triglycerides increase brain energy metabolism in Alzheimer's disease. J Alzheimers Dis. 2018;64:551‐561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

