Cilium axoneme internalization and degradation in chytrid fungi
- PMID: 33103844
- PMCID: PMC7944584
- DOI: 10.1002/cm.21637
Cilium axoneme internalization and degradation in chytrid fungi
Abstract
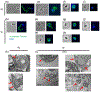
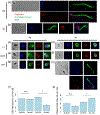
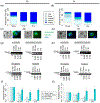
Loss of the cilium is important for cell cycle progression and certain developmental transitions. Chytrid fungi are a group of basal fungi that have retained centrioles and cilia, and they can disassemble their cilia via axoneme internalization as part of the transition from free-swimming spores to sessile sporangia. While this type of cilium disassembly has been observed in many single-celled eukaryotes, it has not been well characterized because it is not observed in common model organisms. To better characterize cilium disassembly via axoneme internalization, we focused on chytrids Rhizoclosmatium globosum and Spizellomyces punctatus to represent two lineages of chytrids with different motility characteristics. Our results show that each chytrid species can reel in its axoneme into the cell body along its cortex on the order of minutes, while S. punctatus has additional faster ciliary compartment loss and lash-around mechanisms. S. punctatus retraction can also occur away from the cell cortex and is partially actin dependent. Post-internalization, the tubulin of the axoneme is degraded in both chytrids over the course of about 2 hr. Axoneme disassembly and axonemal tubulin degradation are both partially proteasome dependent. Overall, chytrid cilium disassembly is a fast process that separates axoneme internalization and degradation.
Keywords: Chytridiomycota; RRID:AB_2535778; RRID:AB_2535809; RRID:AB_2650560; RRID:AB_2715541; RRID:AB_477583; RRID:AB_609894; RRID:AB_621847; RRID:AB_621848; RRID:SCR_000432; RRID:SCR_002285; RRID:SCR_003070; RRID:SCR_016865; axoneme; cilia; proteolysis; tubulin.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors report no conflict of interest.
Figures


References
-
- Antikajian G. (1949). A developmental, morphological, and cytological study of Asterophlyctis with special reference to its sexuality, taxonomy, and relationships. American Journal of Botany, 36(2), 245–262. 10.1002/j.1537-2197.1949.tb05254.x - DOI
-
- Barr DJS (1986). Allochytridium expandens rediscovered: Morphology, physiology and zoospore ultrastructure. Mycologia, 78(3), 439–448. 10.1080/00275514.1986.12025267 - DOI
-
- Barr DJS, & Hartmann VE (1976). Zoospore ultrastructure of three chytridium species and Rhizoclosmatium globosum. Canadian Journal of Botany, 54(17), 2000–2013. 10.1139/b76-214 - DOI

