The circuitry of the tumor microenvironment in adult and pediatric Hodgkin lymphoma: cellular composition, cytokine profile, EBV, and exosomes
- PMID: 33103852
- PMCID: PMC8451374
- DOI: 10.1002/cnr2.1311
The circuitry of the tumor microenvironment in adult and pediatric Hodgkin lymphoma: cellular composition, cytokine profile, EBV, and exosomes
Abstract
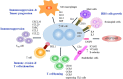
Background: Classical Hodgkin lymphoma (cHL) is a unique lymphoid malignancy with a tumor microenvironment (TME) consisting of a small number of neoplastic-Hodgkin and Reed-Sternberg (H-RS) cells (<1%), surrounded by a large number of nonneoplastic infiltrating immune cells (>90%). The TME of cHL critically depends on immune cells to support tumor growth as H-RS cells cannot survive and proliferate in isolation.
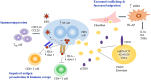
Recent findings: Programmed cell death protein 1 (PD-1) ligand expressed on H-RS cells inhibits the clearance of tumor by causing T-cell exhaustion. Nivolumab and pembrolizumab, PD-1 inhibitors, have been proven to be effective in treating adult and pediatric patients with R/R cHL. Tumor-associated macrophages (TAMs) are a central component of TME and are known to cause poor prognosis in adult HL. However, the prognostic impact of CD68+ TAMs in pediatric HL remains ambiguous. EBV modulates the tumor milieu of HL and plays a strategic role in immune escape by enrichment of the TME with Treg cells and associated immunosuppressive cytokines in adult HL. In contrast, EBV+ pediatric patients have increased infiltration of CD8+ T-cells and show a better therapeutic response suggesting viral-related TME is distinct in childhood HL. The role of CASP3 in apoptosis of H-RS cells and its correlation with response prediction in adult and pediatric HL suggest it may serve as a potential biomarker. In cHL, CD30, EBV, and NF-κB signaling employ exosomes for cell-cell communication that triggers the migration capacity of fibroblasts, stimulate to produce proinflammatory cytokines, and help to create a tumor-supportive microenvironment.
Conclusion: The cHL microenvironment is distinct in adult and pediatric HL. Future studies are required to understand the role of interplay between H-RS cells and EBV-associated microenvironment and their clinical outcome. They may present novel therapeutic targets for the development of antilymphoma therapy.
Keywords: EBV; Hodgkin lymphoma; pediatric; tumor microenvironment.
© 2020 The Authors. Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

