Evidence of Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells from COVID-19 Patients
- PMID: 33103907
- PMCID: PMC7640979
- DOI: 10.1021/acs.jproteome.0c00606
Evidence of Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells from COVID-19 Patients
Abstract
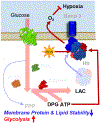
The SARS-CoV-2 beta coronavirus is the etiological driver of COVID-19 disease, which is primarily characterized by shortness of breath, persistent dry cough, and fever. Because they transport oxygen, red blood cells (RBCs) may play a role in the severity of hypoxemia in COVID-19 patients. The present study combines state-of-the-art metabolomics, proteomics, and lipidomics approaches to investigate the impact of COVID-19 on RBCs from 23 healthy subjects and 29 molecularly diagnosed COVID-19 patients. RBCs from COVID-19 patients had increased levels of glycolytic intermediates, accompanied by oxidation and fragmentation of ankyrin, spectrin beta, and the N-terminal cytosolic domain of band 3 (AE1). Significantly altered lipid metabolism was also observed, in particular, short- and medium-chain saturated fatty acids, acyl-carnitines, and sphingolipids. Nonetheless, there were no alterations of clinical hematological parameters, such as RBC count, hematocrit, or mean corpuscular hemoglobin concentration, with only minor increases in mean corpuscular volume. Taken together, these results suggest a significant impact of SARS-CoV-2 infection on RBC structural membrane homeostasis at the protein and lipid levels. Increases in RBC glycolytic metabolites are consistent with a theoretically improved capacity of hemoglobin to off-load oxygen as a function of allosteric modulation by high-energy phosphate compounds, perhaps to counteract COVID-19-induced hypoxia. Conversely, because the N-terminus of AE1 stabilizes deoxyhemoglobin and finely tunes oxygen off-loading and metabolic rewiring toward the hexose monophosphate shunt, RBCs from COVID-19 patients may be less capable of responding to environmental variations in hemoglobin oxygen saturation/oxidant stress when traveling from the lungs to peripheral capillaries and vice versa.
Keywords: AE1; SARS-CoV-2; band 3; erythrocyte; lipidomics; metabolomics; proteomics.
Conflict of interest statement
Figures







Update of
-
Evidence for structural protein damage and membrane lipid remodeling in red blood cells from COVID-19 patients.medRxiv [Preprint]. 2020 Jun 30:2020.06.29.20142703. doi: 10.1101/2020.06.29.20142703. medRxiv. 2020. Update in: J Proteome Res. 2020 Nov 6;19(11):4455-4469. doi: 10.1021/acs.jproteome.0c00606. PMID: 32637980 Free PMC article. Updated. Preprint.
References
-
- Wu F; Zhao S; Yu B; Chen Y-M; Wang W; Song Z-G; Hu Y; Tao Z-W; Tian J-H; Pei Y-Y; Yuan M-L; Zhang Y-L; Dai F-H; Liu Y; Wang Q-M; Zheng J-J; Xu L; Holmes EC; Zhang Y-Z A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579 (7798), 265–269. 10.1038/s41586-020-2008-3. - DOI - PMC - PubMed
-
- Gordon DE; Jang GM; Bouhaddou M; Xu J; Obernier K; O’Meara MJ; Guo JZ; Swaney DL; Tummino TA; Huettenhain R; Kaake RM; Richards AL; Tutuncuoglu B; Foussard H; Batra J; Haas K; Modak M; Kim M; Haas P; Polacco BJ; Braberg H; Fabius JM; Eckhardt M; Soucheray M; Bennett MJ; Cakir M; McGregor MJ; Li Q; Naing ZZC; Zhou Y; Peng S; Kirby IT; Melnyk JE; Chorba JS; Lou K; Dai SA; Shen W; Shi Y; Zhang Z; Barrio-Hernandez I; Memon D; Hernandez-Armenta C; Mathy CJP; Perica T; Pilla KB; Ganesan SJ; Saltzberg DJ; Ramachandran R; Liu X; Rosenthal SB; Calviello L; Venkataramanan S; Liboy-Lugo J; Lin Y; Wankowicz SA; Bohn M; Sharp PP; Trenker R; Young JM; Cavero DA; Hiatt J; Roth TL; Rathore U; Subramanian A; Noack J; Hubert M; Roesch F; Vallet T; Meyer B; White KM; Miorin L; Rosenberg OS; Verba KA; Agard D; Ott M; Emerman M; Ruggero D; García-Sastre A; Jura N; von Zastrow M; Taunton J; Ashworth A; Schwartz O; Vignuzzi M; d’Enfert C; Mukherjee S; Jacobson M; Malik HS; Fujimori DG; Ideker T; Craik CS; Floor S; Fraser JS; Gross J; Sali A; Kortemme T; Beltrao P; Shokat K; Shoichet BK; Krogan NJ A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-Repurposing. bioRxiv 2020, 2020.03.22.002386. 10.1101/2020.03.22.002386. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

