A Palliative Care Intervention for Patients on Phase 1 Studies
- PMID: 33103938
- PMCID: PMC8147498
- DOI: 10.1089/jpm.2020.0597
A Palliative Care Intervention for Patients on Phase 1 Studies
Abstract
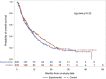
Background: Phase 1 clinical trials remain vital for oncology care. Patients on these trials require supportive care for quality-of-life (QOL) concerns. Objective: To test a Palliative Care Intervention (PCI) for patients with solid tumors enrolled in Phase I therapeutic trials with a priori hypothesis that psychological distress, QOL, satisfaction, symptoms, and resource utilization would be improved in the PCI group. Design: This unblinded randomized trial compared the PCI with usual care in patients accrued to Phase I Clinical Trials. Subjects (n = 479) were followed for 24 weeks, with 12 weeks as the primary outcome. Setting: Two Comprehensive Cancer Centers in the United States. Subjects: A consecutive sample, 21 years or older, English fluency, with solid tumors initiating a Phase 1 trial. Measurements: Psychological Distress (Distress Thermometer), QOL total and subscales (FACT-G), satisfaction (FAM-CARE), survival, and resource utilization (chart audit). Results: PCI subjects showed improved Psychological Distress (-0.47, p = 0.015) and Emotional Well-Being (0.81, p = 0.045), with differences on variables of QOL and distress between sites. High rates of symptom-management admissions (41.3%) and low rates of Advance Directive completion (39.0%), and hospice enrollment (30.7%), despite a median survival in both groups of 10.1 months from initiating a Phase 1 study. Conclusions: A nurse-delivered PCI can improve some QOL outcomes and distress for patients participating in Phase 1 trials. Greater integration of PC is needed to provide quality care to these patients and to support transitions from treatment to supportive care, especially at the end of life. ClinicalTrials.gov Identifier: NCT01612598.
Keywords: palliative care; palliative care intervention; phase 1 clinical trials; quality of life.
Conflict of interest statement
No competing financial interests exist.
Figures
References
-
- Comoretto N, Larumbe A, Arantzamendi M, Centeno C: Palliative care consultants' ethical concerns with advanced cancer patients participating in phase 1 clinical trials: A case study. Prog Palliat Care 2017;25:230–234
-
- Godskesen T, Nygren P, Nordin K, et al. : Phase 1 clinical trials in end-stage cancer: Patient understanding of trial premises and motives for participation. Support Care Cancer 2013;21:3137–3142 - PubMed
-
- Jenkins V, Solis-Trapala I, Langridge C, et al. : What oncologists believe they said and what patients believe they heard: An analysis of phase I trial discussions. J Clin Oncol 2011;29:61–68 - PubMed
-
- Nielsen ZE, Berthelsen CB: Cancer patients' perceptions of factors influencing their decisions on participation in clinical drug trials: A qualitative meta-synthesis. J Clin Nurs 2019;28:2443–2461 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous