Safety and Biovigilance in Organ Donation (SAFEBOD): Protocol for a Population-Based Cohort Study
- PMID: 33104005
- PMCID: PMC7652689
- DOI: 10.2196/18282
Safety and Biovigilance in Organ Donation (SAFEBOD): Protocol for a Population-Based Cohort Study
Abstract
Background: Tension lies between the need to increase access to organ transplantation and the equally urgent need to prevent inadvertent transmission of infectious diseases or cancer from organ donors. Biovigilance, or the evaluation of potential donors, is often time-pressured and may be based on incomplete information.
Objective: The Safety and Biovigilance in Organ Donation (SAFEBOD) study aims to improve estimates of infection and cancer transmission risk and explore how real-time data access could support decision-making.
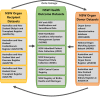
Methods: We will link existing donor referral, actual donor, recipient, and health-outcome data sets from 2000-2015 in New South Wales. Organ donor data sets will include the Organ Donor Characterizing Risk-Profile of Donors Study, the National Organ Matching System, the Australian and New Zealand Organ Donor Register, and the Australian and New Zealand Living Donor Kidney Register. Recipient data sets will include the Australian and New Zealand Dialysis and Transplant Register, the Australian and New Zealand Cardiothoracic Register, the Australian and New Zealand Islet and Pancreas Register, and the Australian and New Zealand Liver Transplant Register. New South Wales health outcome data sets will include HIV and AIDS Notifications and Surveillance Data, the Notifiable Conditions Information Management System, Admitted Patient Data Collection, Emergency Department Data Collection, the Central Cancer Registry, and the Cause of Death Data Collection. We will link organ donors to transplant recipients and health outcomes data sets using probabilistic data-matching based on personal identifiers. Transmission and nontransmission events will be determined by comparing previous cases in donors and posttransplant cases in recipients. We will compare the perceived-risk at referral with the verified risk from linked health outcome data sets and the odds of cancer or contracting an infectious disease in organ recipients from donors based on their transmission-risk profile and estimate recipient survival by donor transmission risk group.
Results: Data were requested from each of the listed registries in September 2018, and data collection is ongoing. Linked data from all listed data sets are expected to be complete in September 2020.
Conclusions: The SAFEBOD study will overcome current limitations in organ donation by accessing comprehensive information on referred organ donors and recipients in existing data sets. The study will provide robust estimates of disease transmission and nontransmission events based on recent data. It will also describe the agreement between perceived risk estimated at the time of referral and verified risk when all health outcome data are accessible. The improved understanding of transmission and nontransmission events will inform clinical decisions and highlight where current policies can be revised to broaden the acceptance of deceased donors.
International registered report identifier (irrid): DERR1-10.2196/18282.
Keywords: biovigilance; cohort study; disease transmission; infectious; neoplasms; organ; organ donor; safety; surgery; transplant; transplant recipients.
©Brenda Rosales, James Hedley, Nicole De La Mata, Claire M Vajdic, Patrick Kelly, Kate Wyburn, Angela C Webster, The SAFEBOD Study Group. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 26.10.2020.
Conflict of interest statement
Conflicts of Interest: The study investigators have no competing interests. All study members have declared all financial and nonfinancial affiliations to the HREC committee in the original ethics application. The data reported here have been supplied by the Australia and New Zealand Dialysis and Transplant Registry. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of ANDATA.
Figures
References
-
- ANZOD Registry . Australia and New Zealand Dialysis and Transplant Registry. Adelaide, Australia: 2018. [2020-09-29]. 2018 Annual Report, Section 2: Deceased Organ. https://www.anzdata.org.au/report/anzod-annual-report-2018/
-
- ANZOD Registry . Australia and New Zealand Dialysis and Transplant Registry. Adelaide, Australia: 2018. [2020-09-29]. 2017 Annual Report, Section 11: Deceased Organ Transplant Waiting List. https://www.anzdata.org.au/report/anzod-annual-report-2018/
-
- Thomson I, Hancock R, O'Leary M, Waller K, Wyburn K, Webster A. 552.9 Risky organs: trends in comorbidities among potential and actual Australian organ donors in New South Wales. Transplantation; 26th International Congress of The Transplantation Society; July 2016, 2016; Hong Kong. 2016.
-
- Avelino-Silva V, D'Albuquerque L, Bonazzi P, Song ATW, Miraglia JL, De Brito Neves A, Abdala E. Liver transplant from Anti-HBc-positive, HBsAg-negative donor into HBsAg-negative recipient: is it safe? A systematic review of the literature. Clin Transplant. 2010;24(6):735–46. doi: 10.1111/j.1399-0012.2010.01254.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources

