A European Observational Study to Evaluate the Safety and the Effectiveness of Safinamide in Routine Clinical Practice: The SYNAPSES Trial
- PMID: 33104040
- PMCID: PMC7990425
- DOI: 10.3233/JPD-202224
A European Observational Study to Evaluate the Safety and the Effectiveness of Safinamide in Routine Clinical Practice: The SYNAPSES Trial
Erratum in
-
A European Observational Study to Evaluate the Safety and the Effectiveness of Safinamide in Routine Clinical Practice: The SYNAPSES Trial.J Parkinsons Dis. 2022;12(1):473. doi: 10.3233/JPD-219007. J Parkinsons Dis. 2022. PMID: 34275911 Free PMC article. No abstract available.
Abstract
Background: Safinamide modulates both dopaminergic and glutamatergic systems with positive effects on motor and non-motor symptoms of Parkinson's disease (PD). The drug utilization study SYNAPSES was designed to investigate the use of safinamide in routine clinical practice, as recommended by the European Medicines Agency.
Objective: To describe the occurrence of adverse events in PD patients treated with safinamide in real-life conditions.
Methods: The SYNAPSES trial is an observational, European, multicenter, retrospective-prospective cohort study. Patients were followed up to 12 months with analyses performed in the overall population and in patients aged >75 years, with relevant comorbidities and with psychiatric conditions.
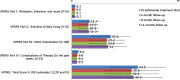
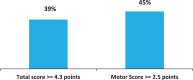
Results: Of the 1610 patients included, 82.4% were evaluable after 12 months with 25.1% of patients >75 years, 70.8% with relevant comorbidities and 42.4% with psychiatric conditions. During observation 45.8% patients experienced adverse events, 27.7% patients had adverse drug reactions and 9.2% patients had serious adverse events. The adverse events were those already described in the patients' information leaflet. The majority were mild or moderate and completely resolved and no differences were detected between the subgroup of patients. Clinically significant improvements were seen in the UPDRS motor score and in the UPDRS total score in ≥40% of patients, according to the criteria developed by Shulman et al.Conclusion:The SYNAPSES study confirms the good safety profile of safinamide even in special groups of patients. Motor complications and motor scores improved with clinically significant results in the UPDRS scale maintained in the long-term.
Keywords: MAO-B inhibitor; Parkinson’s disease; real-life evaluation; safinamide.
Conflict of interest statement
SYNAPSES Study Investigators Group
Belgium: Bergmans B, Bourgeois P, Cras P, De Klippel N, Dethy S, Franco G, Garraux G, Geens K, Jacquerye P, Jeanjean A, Santens P, Supiot F, Van der Linden C.
Germany: Blersch WK, Delf M, Hellwig B, Herbst HP, Kupsch A, Jost WH, Lang M, Muhlack S, Nastos I, Oehlwein C, Schlegel E, Schwarz J, Warnecke T, Woitalla D.
Italy: Abbruzzese G, Aguggia M, Avarello T, Barone P, Baruffaldi R, Belgrado E, Bentivoglio AR, Bosco D, Calabresi P, Callegarini C, Cannas A, Centonze D, Ceravolo R, Colosimo C, Comi C, Contardi S, Cortelli P, Cossu G, D’Amelio M, De Pandis MF, Denaro A, Di Lazzaro V, Fabbrini G, Gasparoli E, Guidi M, Iliceto G, Lopiano L, Manganotti P, Marconi R, Marini C, Marsala SZ, Mauri M, Moleri M, Monge A, Morgante F, Negrotti A, Nordera G, Onofrj M, Pacchetti C, Padovani A, Pontieri FE, Priori A, Quatrale R, Sensi M, Stefani A, Tamma F, Tessitore A, Tinazzi M, Vitale C, Volontè MA, Zappia M, Zecchinelli AL.
Spain: Arbelo Gonzalez JM, Bayés A, Blazquez M, Calopa Garriga M, Callen A, Campos Arillo V, Cubo E, De Fábregues O, Escalante Arroyo S, Espinosa Rosso R, Esquivel López A, Freire E, García Cobos E, García Moreno JM, Gomez-Esteban JC, González-Ardura J, Grandas Perez F, Kulisevsky J, Kurtis M, Juni J, Legarda I, Leiva C, López Aristegui N, López Manzanares L, Lozano JJ, Luquín MR, Martinez Castrillo JC, Martí Domenech MJ, Martínez I, Mata M, Mir Rivera P, Pascual Sedano B, Rodríguez Oroz MC, Rodríguez Uranga JJ, Sanchez S, Santos García D, Solano B, Vaamonde Gamo J.
Switzerland: Accolla E, Bohlhalter S, Kälin A, Kägi G, Michelis J.
U.K.: Carrol C, Henderson E, Raha S, Raw J, Silva N, Silverdale M.
Figures


References
-
- de Lau LM, Giesbergen PC, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MM (2004) Incidence of parkinsonism and Parkinson’s Disease in a general population: The Rotterdam Study. Neurology 63, 1240–1244. - PubMed
-
- Chaudhuri KR, Shapira AH (2009) Non-motor symptoms of Parkinson’s disease: Dopaminergic pathopshysiology and treatment. Lancet Neurol 8, 464–474. - PubMed
-
- Hauser RA (2009) Levodopa: Past, present and future. Eur Neurol 62, 1–8. - PubMed
-
- Chase TN, Bibbiani F, Oh JD (2003) Striatal glutamatergic mechanisms and extrapyramidal movement disorders. Neurotox Res 5, 139–146. - PubMed
-
- Müller T, Foley P (2017) Clinical pharmacokinetics and pharmacodynamics of safinamide. Clin Pharmacokinet 56, 251–261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

