Isolation, Transfection, and Long-Term Culture of Adult Mouse and Rat Cardiomyocytes
- PMID: 33104067
- PMCID: PMC8276663
- DOI: 10.3791/61073
Isolation, Transfection, and Long-Term Culture of Adult Mouse and Rat Cardiomyocytes
Abstract
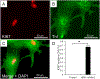
Ex vivo culture of the adult mammalian cardiomyocytes (CMs) presents the most relevant experimental system for the in vitro study of cardiac biology. Adult mammalian CMs are terminally differentiated cells with minimal proliferative capacity. The post-mitotic state of adult CMs not only restricts cardiomyocyte cell cycle progression but also limits the efficient culture of CMs. Moreover, the long-term culture of adult CMs is necessary for many studies, such as CM proliferation and analysis of gene expression. The mouse and the rat are the two most preferred laboratory animals to be used for cardiomyocyte isolation. While the long-term culture of rat CMs is possible, adult mouse CMs are susceptible to death and cannot be cultured more than five days under normal conditions. Therefore, there is a critical need to optimize the cell isolation and long-term culture protocol for adult murine CMs. With this modified protocol, it is possible to successfully isolate and culture both adult mouse and rat CMs for more than 20 days. Moreover, the siRNA transfection efficiency of isolated CM is significantly increased compared to previous reports. For adult mouse CM isolation, the Langendorff perfusion method is utilized with an optimal enzyme solution and sufficient time for complete extracellular matrix dissociation. In order to obtain pure ventricular CMs, both atria were dissected and discarded before proceeding with the disassociation and plating. Cells were dispersed on a laminin coated plate, which allowed for efficient and rapid attachment. CMs were allowed to settle for 4-6 h before siRNA transfection. Culture media was refreshed every 24 h for 20 days, and subsequently, CMs were fixed and stained for cardiac-specific markers such as Troponin and markers of cell cycle such as KI67.
Figures




References
-
- Gonzalez-Rosa JM, Martin V, Peralta M, Torres M, Mercader N Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development. 138, 1663–1674 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
