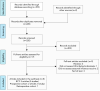
Summary of the NACI systematic review and recommendation on the use of live attenuated influenza vaccine (LAIV) in HIV-infected individuals
- PMID: 33104088
- PMCID: PMC7556201
- DOI: 10.14745/ccdr.v46i09a08
Summary of the NACI systematic review and recommendation on the use of live attenuated influenza vaccine (LAIV) in HIV-infected individuals
Abstract
Background: Annual influenza vaccination is recommended for all individuals six months of age and older, including those with HIV infection. Prior to this statement, the National Advisory Committee on Immunization (NACI) stated that live attenuated influenza vaccine (LAIV) was contraindicated for all individuals with HIV infection. The objective of this article is to update NACI's guidance on the use of LAIV for HIV-infected individuals.
Methods: A systematic literature review of the use of LAIV in individuals with HIV was undertaken. The Canadian Adverse Events Following Immunization Surveillance System was searched for reports of adverse events following vaccination with LAIV in HIV-infected individuals. NACI approved the revised recommendations.
Results: NACI concluded that LAIV is immunogenic in children with HIV, and available data suggest that it is safe, although data were insufficient to detect possible uncommon adverse effects. LAIV may be considered as an option for vaccination of children 2-17 years old who meet the following criteria: 1) receiving highly active antiretroviral therapy for at least four months; 2) CD4 count of 500/µL or greater if age 2-5 years, or of 200/µL or greater if age 6-17 years; and 3) HIV plasma RNA less than 10,000 copies/mL. LAIV remains contraindicated for adults with HIV because of insufficient data. Intramuscular influenza vaccination is considered the standard for children living with HIV by NACI and the Canadian Paediatric & Perinatal HIV/AIDS Research Group, particularly for those without HIV viral load suppression (i.e. plasma HIV RNA is 40 copies/mL or greater). However, if intramuscular (IM) vaccination is not accepted by the patient or substitute decision-maker, LAIV would be reasonable for children meeting the criteria listed above.
Conclusion: LAIV may be considered as an option for annual vaccination of selected children with HIV.
Keywords: : National Advisory Committee on Immunization; HIV; NACI; literature review; live attenuated influenza vaccine.
Conflict of interest statement
Competing interests: None.
Figures
Similar articles
-
Summary of the NACI Seasonal Influenza Vaccine Statement for 2020-2021.Can Commun Dis Rep. 2020 May 7;46(5):132-137. doi: 10.14745/ccdr.v46i05a06. eCollection 2020 May 7. Can Commun Dis Rep. 2020. PMID: 32558810 Free PMC article.
-
Summary of the National Advisory Committee on Immunization (NACI) Statement on Seasonal Influenza Vaccine for 2016-2017.Can Commun Dis Rep. 2016 Sep 1;42(9):188-192. doi: 10.14745/ccdr.v42i09a06. eCollection 2016 Sep 1. Can Commun Dis Rep. 2016. PMID: 29770030 Free PMC article.
-
Summary of the National Advisory Committee on Immunization (NACI) Statement on Seasonal Influenza Vaccine for 2015-2016.Can Commun Dis Rep. 2015 Oct 1;41(10):227-232. doi: 10.14745/ccdr.v41i10a02. eCollection 2015 Oct 1. Can Commun Dis Rep. 2015. PMID: 29769917 Free PMC article.
-
Live Attenuated Influenza Vaccine: Is Past Performance a Guarantee of Future Results?Clin Ther. 2018 Aug;40(8):1246-1254. doi: 10.1016/j.clinthera.2018.07.003. Epub 2018 Aug 6. Clin Ther. 2018. PMID: 30093132 Review.
-
Seasonal influenza vaccines.Curr Top Microbiol Immunol. 2009;333:43-82. doi: 10.1007/978-3-540-92165-3_3. Curr Top Microbiol Immunol. 2009. PMID: 19768400 Review.
References
-
- National Advisory Committee on Immunization. Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2019–2020. Ottawa (ON): NACI; 2019 (accessed 2020-02-17). https://www.canada.ca/en/public-health/services/publications/vaccines-im...
-
- National Advisory Committee on Immunization. Recommendations on the use of live, attenuated influenza vaccine (FluMist®). Supplemental Statement on Seasonal Influenza Vaccine for 2011-2012. An Advisory Committee Statement (ACS). Can Commun Dis Rep. 2011;37(ACS-7):1-77. 10.14745/ccdr.v37i00a07 - DOI - PMC - PubMed
-
- AstraZeneca. Product Monograph: FluMist® Quadrivalent. 2018 (accessed 2020-02-17). https://pdf.hres.ca/dpd_pm/00047438.PDF
-
- U.S. Department of Health and Human Services. AIDS Info. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Exposed and HIV-Infected Children (accessed 2020-02-17). https://aidsinfo.nih.gov/guidelines/brief-html/5/pediatric-opportunistic...
-
- BC Centre for Disease Control. Human Immunodeficiency Virus (HIV) Infection. BCCDC; 2018 (accessed 2020-02-17). http://www.bccdc.ca/resource-gallery/Documents/Guidelines%20and%20Forms/...
LinkOut - more resources
Full Text Sources
Research Materials