Bridging the gap between natural product synthesis and drug discovery
- PMID: 33104139
- PMCID: PMC7718793
- DOI: 10.1039/d0np00048e
Bridging the gap between natural product synthesis and drug discovery
Abstract
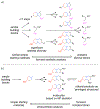
Covering: 1986 to 2020Natural products are an enduring source of chemical information useful for probing biologically relevant chemical space. Toward gathering further structure-activity relationship (SAR) information for a particular natural product, synthetic chemists traditionally proceeded first by a total synthesis effort followed by the synthesis of simplified derivatives. While this approach has proven fruitful, it often does not incorporate hypotheses regarding structural features necessary for bioactivity at the synthetic planning stage, but rather focuses on the rapid assembly of the targeted natural product; a goal that often supersedes the opportunity to gather SAR information en route to the natural product. Furthermore, access to simplified variants of a natural product possessing only the proposed essential structural features necessary for bioactivity, typically at lower oxidation states overall, is sometimes non-trivial from the original established synthetic route. In recent years, several synthetic design strategies were described to streamline the process of finding bioactive molecules in concert with fathering further SAR studies for targeted natural products. This review article will briefly discuss traditional retrosynthetic strategies and contrast them to selected examples of recent synthetic strategies for the investigation of biologically relevant chemical space revealed by natural products. These strategies include: diversity-oriented synthesis (DOS), biology-oriented synthesis (BIOS), diverted-total synthesis (DTS), analogue-oriented synthesis (AOS), two-phase synthesis, function-oriented synthesis (FOS), and computed affinity/dynamically ordered retrosynthesis (CANDOR). Finally, a description of pharmacophore-directed retrosynthesis (PDR) developed in our laboratory and initial applications will be presented that was initially inspired by a retrospective analysis of our synthetic route to pateamine A completed in 1998.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures


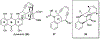


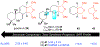


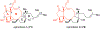








References
-
- Wöhler F, Ann. Phys. (Berlin), 1828, 87, 253–256.
-
- Corey EJ and Cheng XM, The Logic of Chemical Synthesis, Wiley, 1989.
-
- Hendrickson JB, J. Am. Chem. Soc, 1975, 97, 5784–5800.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

