MAVS regulates the quality of the antibody response to West-Nile Virus
- PMID: 33104760
- PMCID: PMC7644103
- DOI: 10.1371/journal.ppat.1009009
MAVS regulates the quality of the antibody response to West-Nile Virus
Abstract
A key difference that distinguishes viral infections from protein immunizations is the recognition of viral nucleic acids by cytosolic pattern recognition receptors (PRRs). Insights into the functions of cytosolic PRRs such as the RNA-sensing Rig-I-like receptors (RLRs) in the instruction of adaptive immunity are therefore critical to understand protective immunity to infections. West Nile virus (WNV) infection of mice deficent of RLR-signaling adaptor MAVS results in a defective adaptive immune response. While this finding suggests a role for RLRs in the instruction of adaptive immunity to WNV, it is difficult to interpret due to the high WNV viremia, associated exessive antigen loads, and pathology in the absence of a MAVS-dependent innate immune response. To overcome these limitations, we have infected MAVS-deficient (MAVSKO) mice with a single-round-of-infection mutant of West Nile virus. We show that MAVSKO mice failed to produce an effective neutralizing antibody response to WNV despite normal antibody titers against the viral WNV-E protein. This defect occurred independently of antigen loads or overt pathology. The specificity of the antibody response in infected MAVSKO mice remained unchanged and was still dominated by antibodies that bound the neutralizing lateral ridge (LR) epitope in the DIII domain of WNV-E. Instead, MAVSKO mice produced IgM antibodies, the dominant isotype controlling primary WNV infection, with lower affinity for the DIII domain. Our findings suggest that RLR-dependent signals are important for the quality of the humoral immune response to WNV.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




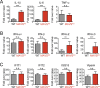

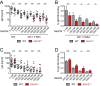
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

