Diagnostic Criteria for Differentiated Vulvar Intraepithelial Neoplasia and Vulvar Aberrant Maturation
- PMID: 33105449
- PMCID: PMC7748053
- DOI: 10.1097/LGT.0000000000000572
Diagnostic Criteria for Differentiated Vulvar Intraepithelial Neoplasia and Vulvar Aberrant Maturation
Abstract
Objective: The aim of the study was to describe the features required for diagnosis of differentiated vulvar intraepithelial neoplasia (dVIN) and vulvar aberrant maturation (VAM).
Materials and methods: The International Society of the Study of Vulvovaginal Diseases tasked the difficult pathologic diagnoses committee to develop consensus recommendations for clinicopathologic diagnosis of vulvar lichen planus, lichen sclerosus, and dVIN. The dVIN subgroup reviewed the literature and formulated diagnostic criteria that were reviewed by the committee and then approved by the International Society of the Study of Vulvovaginal Diseases membership.
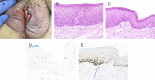
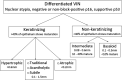
Results: Differentiated vulvar intraepithelial neoplasia is the immediate precursor of human papillomavirus (HPV)-independent vulvar squamous cell carcinoma and shows a spectrum of clinical and microscopic appearances, some overlapping with HPV-related neoplasia. The histopathologic definition of dVIN is basal atypia combined with negative or nonblock-positive p16 and basal overexpressed, aberrant negative, or wild-type p53. The most common pattern of dVIN is keratinizing with acanthosis, aberrant rete ridge pattern, and premature maturation. The morphologic spectrum of keratinizing dVIN includes hypertrophic, atrophic, acantholytic, and subtle forms. A few dVIN cases are nonkeratinizing, with basaloid cells replacing more than 60% of epithelium. Vulvar aberrant maturation is an umbrella term for lesions with aberrant maturation that arise out of lichenoid dermatitis and lack the basal atypia required for dVIN.
Conclusions: Evaluation of women at risk for dVIN and VAM requires a collaborative approach by clinicians and pathologists experienced in vulvar disorders. Close surveillance of women with lichen sclerosus and use of these recommendations may assist in prevention of HPV-independent squamous cell carcinoma through detection and treatment of dVIN and VAM.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the ASCCP.
Conflict of interest statement
The authors have declared they have no conflicts of interest.
Figures







References
-
- Eva LJ Sadler L Fong KL, et al. . Trends in HPV-dependent and HPV-independent vulvar cancers: the changing face of vulvar squamous cell carcinoma. Gynecol Oncol 2020;157:450–5. - PubMed
-
- de Sanjosé S Alemany L Ordi J, et al. , HPV VVAP study group . Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer 2013;49:3450–61. - PubMed
-
- van de Nieuwenhof HP Massuger LF van der Avoort IA, et al. . Vulvar squamous cell carcinoma development after diagnosis of VIN increases with age. Eur J Cancer 2009;45:851–6. - PubMed
-
- Hinten F Molijn A Eckhardt L, et al. . Vulvar cancer: two pathways with different localization and prognosis. Gynecol Oncol 2018;149:310–7. - PubMed
-
- Kashofer K, Regauer S. Analysis of full coding sequence of the TP53 gene in invasive vulvar cancers: implications for therapy. Gynecol Oncol 2017;146:314–8. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

