A Markov chain approach for ranking treatments in network meta-analysis
- PMID: 33105517
- PMCID: PMC7821202
- DOI: 10.1002/sim.8784
A Markov chain approach for ranking treatments in network meta-analysis
Abstract
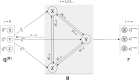
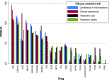
When interpreting the relative effects from a network meta-analysis (NMA), researchers are usually aware of the potential limitations that may render the results for some comparisons less useful or meaningless. In the presence of sufficient and appropriate data, some of these limitations (eg, risk of bias, small-study effects, publication bias) can be taken into account in the statistical analysis. Very often, though, the necessary data for applying these methods are missing and data limitations cannot be formally integrated into ranking. In addition, there are other important characteristics of the treatment comparisons that cannot be addressed within a statistical model but only through qualitative judgments; for example, the relevance of data to the research question, the plausibility of the assumptions, and so on. Here, we propose a new measure for treatment ranking called the Probability of Selecting a Treatment to Recommend (POST-R). We suggest that the order of treatments should represent the process of considering treatments for selection in clinical practice and we assign to each treatment a probability of being selected. This process can be considered as a Markov chain model that allows the end-users of NMA to select the most appropriate treatments based not only on the NMA results but also to information external to the NMA. In this way, we obtain rankings that can inform decision-making more efficiently as they represent not only the relative effects but also their potential limitations. We illustrate our approach using a NMA comparing treatments for chronic plaque psoriasis and we provide the Stata commands.
Keywords: comparative effectiveness research; multiple treatments; selection probabilities; stochastic process; treatment hierarchy.
© 2020 The Authors. Statistics in Medicine published by John Wiley & Sons Ltd.
Figures


References
-
- Trinquart L, Attiche N, Bafeta A, Porcher R, Ravaud P. Uncertainty in treatment rankings: reanalysis of network meta‐analyses of randomized trials. Ann Intern Med. 2016;164(10):666‐673. - PubMed
-
- Veroniki AA, Straus SE, Rücker G, Tricco AC. Commentary: is providing uncertainty intervals in treatment ranking helpful in a network meta‐analysis? J Clin Epidemiol. 2018;100:122‐129. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
