Effect of Pioglitazone on Perihematomal Edema in Intracerebral Hemorrhage Mouse Model by Regulating NLRP3 Expression and Energy Metabolism
- PMID: 33105536
- PMCID: PMC7671775
- DOI: 10.3340/jkns.2020.0056
Effect of Pioglitazone on Perihematomal Edema in Intracerebral Hemorrhage Mouse Model by Regulating NLRP3 Expression and Energy Metabolism
Abstract
Objective: Cerebral edema is the predominant mechanism of secondary inflammation after intracerebral hemorrhage (ICH). Pioglitazone, peroxisome proliferator-activated receptor gamma agonist has been shown to play a role in regulation of central nervous system inflammation. Here, we examined the pharmacological effects of pioglitazone in an ICH mouse model and investigated its regulation on NLRP3 inflammasome and glucose metabolism.
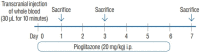
Methods: The ICH model was established in C57 BL/6 mice by the stereotactical inoculation of blood (30 µL) into the right frontal lobe. The treatment group was administered i.p. pioglitazone (20 mg/kg) for 1, 3, and 6 days. The control group was administered i.p. phosphate-buffered saline for 1, 3, and 6 days. We investigated brain water contents, NLRP3 expression, and changes in the metabolites in the ICH model using liquid chromatography-tandem mass spectrometry.
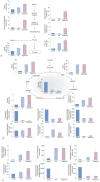
Results: On day 3, brain edema in the mice treated with pioglitazone was decreased more than that in the control group. Expression levels of NLRP3 in the ICH model treated with pioglitazone were decreased more than those of the control mice on days 3 and 7. The pioglitazone group showed higher levels of glycolytic metabolites than those in the ICH mice. Lactate production was increased in the ICH mice treated with pioglitazone.
Conclusion: Our results demonstrated less brain swelling following ICH in mice treated with pioglitazone. Pioglitazone decreased NLRP3-related brain edema and increased anaerobic glycolysis, resulting in the production of lactate in the ICH mice model. NLRP3 might be a therapeutic target for ICH recovery.
Keywords: Brain edema; Cerebral hemorrhage; Inflammasomes; Lactates; Pioglitazone.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures



Similar articles
-
Cordycepin confers neuroprotection in mice models of intracerebral hemorrhage via suppressing NLRP3 inflammasome activation.Metab Brain Dis. 2017 Aug;32(4):1133-1145. doi: 10.1007/s11011-017-0003-7. Epub 2017 Apr 11. Metab Brain Dis. 2017. PMID: 28401330
-
The role of NLRP3 in traumatic brain injury and its regulation by pioglitazone.J Neurosurg. 2019 Sep 27;133(4):1083-1091. doi: 10.3171/2019.6.JNS1954. Print 2020 Oct 1. J Neurosurg. 2019. PMID: 31561220
-
Selective NLRP3 (Pyrin Domain-Containing Protein 3) Inflammasome Inhibitor Reduces Brain Injury After Intracerebral Hemorrhage.Stroke. 2018 Jan;49(1):184-192. doi: 10.1161/STROKEAHA.117.018904. Epub 2017 Dec 6. Stroke. 2018. PMID: 29212744 Free PMC article.
-
Brain edema after intracerebral hemorrhage in rats: the role of iron overload and aquaporin 4.J Neurosurg. 2009 Mar;110(3):462-8. doi: 10.3171/2008.4.JNS17512. J Neurosurg. 2009. PMID: 19025353
-
Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-κB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway.J Neuroinflammation. 2017 Jun 13;14(1):119. doi: 10.1186/s12974-017-0895-5. J Neuroinflammation. 2017. PMID: 28610608 Free PMC article.
Cited by
-
Molecular, Pathological, Clinical, and Therapeutic Aspects of Perihematomal Edema in Different Stages of Intracerebral Hemorrhage.Oxid Med Cell Longev. 2022 Sep 17;2022:3948921. doi: 10.1155/2022/3948921. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36164392 Free PMC article. Review.
-
Brain edema formation and therapy after intracerebral hemorrhage.Neurobiol Dis. 2023 Jan;176:105948. doi: 10.1016/j.nbd.2022.105948. Epub 2022 Dec 5. Neurobiol Dis. 2023. PMID: 36481437 Free PMC article. Review.
-
Interplay between Gut Microbiota and NLRP3 Inflammasome in Intracerebral Hemorrhage.Nutrients. 2022 Dec 9;14(24):5251. doi: 10.3390/nu14245251. Nutrients. 2022. PMID: 36558410 Free PMC article. Review.
-
A new perspective on the regulation of neuroinflammation in intracerebral hemorrhage: mechanisms of NLRP3 inflammasome activation and therapeutic strategies.Front Immunol. 2025 Feb 27;16:1526786. doi: 10.3389/fimmu.2025.1526786. eCollection 2025. Front Immunol. 2025. PMID: 40083546 Free PMC article. Review.
-
Targeting the NLRP3-ROS Axis: Disrupting the Oxidative-Inflammatory Vicious Cycle in Intracerebral Hemorrhage.J Inflamm Res. 2025 Jul 24;18:9849-9870. doi: 10.2147/JIR.S529884. eCollection 2025. J Inflamm Res. 2025. PMID: 40735267 Free PMC article. Review.
References
-
- Alessandri B, Schwandt E, Kamada Y, Nagata M, Heimann A, Kempski O. The neuroprotective effect of lactate is not due to improved glutamate uptake after controlled cortical impact in rats. J Neurotrauma. 2012;29:2181–2191. - PubMed
-
- Au A. Metabolomics and lipidomics of ischemic stroke. Adv Clin Chem. 2018;85:31–69. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

