Soluble Compounds Released by Hypoxic Stroma Confer Invasive Properties to Pancreatic Ductal Adenocarcinoma
- PMID: 33105540
- PMCID: PMC7690284
- DOI: 10.3390/biomedicines8110444
Soluble Compounds Released by Hypoxic Stroma Confer Invasive Properties to Pancreatic Ductal Adenocarcinoma
Abstract
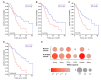
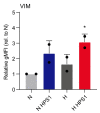
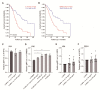
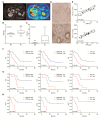
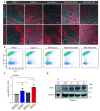
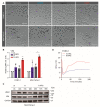
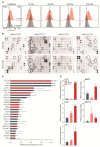
Pancreatic ductal adenocarcinoma (PDAC) is characterized by abundant stroma and a hypoxic microenvironment. Pancreatic stellate cells (PSC) are activated by hypoxia and promote excessive desmoplasia, further contributing to the development of hypoxia. We aimed to explore how hypoxia and stroma interact to contribute to invasive growth in PDAC. [18F]HX4 PET/CT was found to be a feasible non-invasive method to assess tumor hypoxia in 42 patients and correlated with HIF1α immunohistochemistry in matched surgical specimens. [18F]HX4 uptake and HIF1α were strong prognostic markers for overall survival. Co-culture and medium transfer experiments demonstrated that hypoxic PSCs and their supernatant induce upregulation of mesenchymal markers in tumor cells, and that hypoxia-induced stromal factors drive invasive growth in hypoxic PDACs. Through stepwise selection, stromal MMP10 was identified as the most likely candidate responsible for this. In conclusion, hypoxia-activated PSCs promote the invasiveness of PDAC through paracrine signaling. The identification of PSC-derived MMP10 may provide a lead to develop novel stroma-targeting therapies.
Keywords: epithelial-to-mesenchymal transition; hypoxia; pancreatic ductal adenocarcinoma; pancreatic stellate cells; stroma.
Conflict of interest statement
HWML has acted as a consultant for BMS, Eli Lilly and Company, MSD, Nordic Pharma Group/Taiho, and Servier, and has received unrestricted research grants from Amgen, Bayer Schering Pharma AG, BMS, Celgene, Eli Lilly and Company, GlaxoSmithKline Pharmaceuticals, MSD, Nordic Pharma Group, Philips, and Roche Pharmaceuticals. MFB has received research funding from Celgene and acted as a consultant to Servier. MGB has received research funding form Ethicon, Medtronic, and Mylan. None of these companies were involved in the design, conduct, or analysis of this study or drafting of the manuscript and decision to publish.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

