Prebiotic Xylo-Oligosaccharides Ameliorate High-Fat-Diet-Induced Hepatic Steatosis in Rats
- PMID: 33105554
- PMCID: PMC7690286
- DOI: 10.3390/nu12113225
Prebiotic Xylo-Oligosaccharides Ameliorate High-Fat-Diet-Induced Hepatic Steatosis in Rats
Abstract
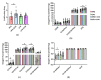
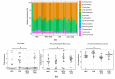
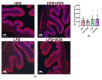
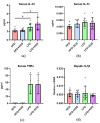
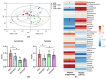
Understanding the importance of the gut microbiota (GM) in non-alcoholic fatty liver disease (NAFLD) has raised the hope for therapeutic microbes. We have shown that high hepatic fat content associated with low abundance of Faecalibacterium prausnitzii in humans and, further, the administration of F. prausnitzii prevented NAFLD in mice. Here, we aimed at targeting F. prausnitzii by prebiotic xylo-oligosaccharides (XOS) to treat NAFLD. First, the effect of XOS on F. prausnitzii growth was assessed in vitro. Then, XOS was supplemented or not with high (HFD, 60% of energy from fat) or low (LFD) fat diet for 12 weeks in Wistar rats (n = 10/group). XOS increased F. prausnitzii growth, having only a minor impact on the GM composition. When supplemented with HFD, XOS ameliorated hepatic steatosis. The underlying mechanisms involved enhanced hepatic β-oxidation and mitochondrial respiration. Nuclear magnetic resonance (1H-NMR) analysis of cecal metabolites showed that, compared to the HFD, the LFD group had a healthier cecal short-chain fatty acid profile and on the HFD, XOS reduced cecal isovalerate and tyrosine, metabolites previously linked to NAFLD. Cecal branched-chain fatty acids associated positively and butyrate negatively with hepatic triglycerides. In conclusion, XOS supplementation can ameliorate NAFLD by improving hepatic oxidative metabolism and affecting GM.
Keywords: fatty liver; gut microbiota; metabolism; mitochondria; oligosaccharides; prebiotic.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
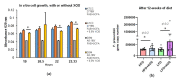






Similar articles
-
Effects of xylo-oligosaccharide on gut microbiota, brain protein expression, and lipid profile induced by high-fat diet.J Nutr Biochem. 2024 Jul;129:109640. doi: 10.1016/j.jnutbio.2024.109640. Epub 2024 Apr 5. J Nutr Biochem. 2024. PMID: 38583497
-
Xylo-Oligosaccharides in Prevention of Hepatic Steatosis and Adipose Tissue Inflammation: Associating Taxonomic and Metabolomic Patterns in Fecal Microbiomes with Biclustering.Int J Environ Res Public Health. 2021 Apr 12;18(8):4049. doi: 10.3390/ijerph18084049. Int J Environ Res Public Health. 2021. PMID: 33921370 Free PMC article.
-
Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens.Poult Sci. 2018 Mar 1;97(3):874-881. doi: 10.3382/ps/pex372. Poult Sci. 2018. PMID: 29294100
-
A systematic review and meta-analysis of randomized controlled trials: effects of mediterranean diet and low-fat diet on liver enzymes and liver fat content of NAFLD.Food Funct. 2024 Aug 12;15(16):8248-8257. doi: 10.1039/d4fo01461h. Food Funct. 2024. PMID: 39076035
-
A review of the capacity of xylooligosaccharides to modulate gut microbiota and promote health.Food Funct. 2025 Jun 16;16(12):4654-4672. doi: 10.1039/d5fo00169b. Food Funct. 2025. PMID: 40470881 Review.
Cited by
-
Branched-chain amino acid supplementation and voluntary running have distinct effects on the serum metabolome of rats with high or low intrinsic aerobic capacity.Front Nutr. 2024 Nov 19;11:1450386. doi: 10.3389/fnut.2024.1450386. eCollection 2024. Front Nutr. 2024. PMID: 39628463 Free PMC article.
-
Gut microbiota-mitochondrial inter-talk in non-alcoholic fatty liver disease.Front Nutr. 2022 Sep 20;9:934113. doi: 10.3389/fnut.2022.934113. eCollection 2022. Front Nutr. 2022. PMID: 36204383 Free PMC article. Review.
-
Estrogen deficiency reduces maximal running capacity and affects serotonin levels differently in the hippocampus and nucleus accumbens in response to acute exercise.Front Neurosci. 2024 Jun 25;18:1399229. doi: 10.3389/fnins.2024.1399229. eCollection 2024. Front Neurosci. 2024. PMID: 38983274 Free PMC article.
-
Oligosaccharides improve the flesh quality and nutrition value of Nile tilapia fed with high carbohydrate diet.Food Chem (Oxf). 2021 Sep 13;3:100040. doi: 10.1016/j.fochms.2021.100040. eCollection 2021 Dec 30. Food Chem (Oxf). 2021. PMID: 35415664 Free PMC article.
-
Dietary Addition With Clostridium butyricum and Xylo-Oligosaccharides Improves Carcass Trait and Meat Quality of Huanjiang Mini-Pigs.Front Nutr. 2021 Nov 5;8:748647. doi: 10.3389/fnut.2021.748647. eCollection 2021. Front Nutr. 2021. PMID: 34805243 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

