Targeted Molecular Therapies in the Treatment of Esophageal Adenocarcinoma, Are We There Yet?
- PMID: 33105560
- PMCID: PMC7690268
- DOI: 10.3390/cancers12113077
Targeted Molecular Therapies in the Treatment of Esophageal Adenocarcinoma, Are We There Yet?
Abstract
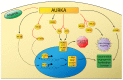
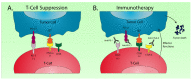
Esophageal adenocarcinoma is one of the leading causes of cancer-related deaths worldwide. The incidence of esophageal adenocarcinoma has increased at an alarming rate in the Western world and long-term survival remains poor. Current treatment approaches involve a combination of surgery, chemotherapy, and radiotherapy. Unfortunately, standard first-line approaches are met with high rates of recurrence and metastasis. More recent investigations into the distinct molecular composition of these tumors have uncovered key genetic and epigenetic alterations involved in tumorigenesis and progression. These discoveries have driven the development of targeted therapeutic agents in esophageal adenocarcinoma. While many agents have been studied, therapeutics targeting the human epidermal growth factor receptor (HER2) and vascular endothelial growth factor (VEGF) pathways have demonstrated improved survival. More recent advances in immunotherapies have also demonstrated survival advantages with monoclonal antibodies targeting the programmed death ligand 1 (PD-L1). In this review we highlight recent advances of targeted therapies, specifically agents targeting receptor tyrosine kinases, small molecule kinase inhibitors, and immune checkpoint inhibitors. While targeted therapeutics and immunotherapies have significantly improved survival, the benefits are limited to patients whose tumors express biomarkers such as PD-L1 and HER2. Survival remains poor for the remainder of patients with esophageal adenocarcinoma, underscoring the critical need for development of novel treatment strategies.
Keywords: esophageal adenocarcinoma; immunotherapy; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Howlader N., Noone A.M., Krapcho M., Miller D., Brest A., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., et al. SEER Cancer Statistics Review, 1975–2016. National Cancer Institute; Bethesda, MD, USA: 2020. [(accessed on 21 May 2020)]. Available online: www.seer.cancer.gov/archive/csr/1975_2016/
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

