Preparation of Defined Chitosan Oligosaccharides Using Chitin Deacetylases
- PMID: 33105791
- PMCID: PMC7660110
- DOI: 10.3390/ijms21217835
Preparation of Defined Chitosan Oligosaccharides Using Chitin Deacetylases
Abstract
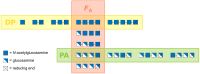
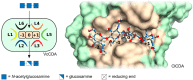
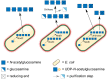
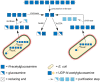
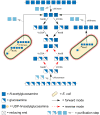
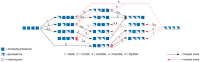
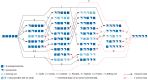
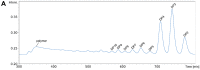
During the past decade, detailed studies using well-defined 'second generation' chitosans have amply proved that both their material properties and their biological activities are dependent on their molecular structure, in particular on their degree of polymerisation (DP) and their fraction of acetylation (FA). Recent evidence suggests that the pattern of acetylation (PA), i.e., the sequence of acetylated and non-acetylated residues along the linear polymer, is equally important, but chitosan polymers with defined, non-random PA are not yet available. One way in which the PA will influence the bioactivities of chitosan polymers is their enzymatic degradation by sequence-dependent chitosan hydrolases present in the target tissues. The PA of the polymer substrates in conjunction with the subsite preferences of the hydrolases determine the type of oligomeric products and the kinetics of their production and further degradation. Thus, the bioactivities of chitosan polymers will at least in part be carried by the chitosan oligomers produced from them, possibly through their interaction with pattern recognition receptors in target cells. In contrast to polymers, partially acetylated chitosan oligosaccharides (paCOS) can be fully characterised concerning their DP, FA, and PA, and chitin deacetylases (CDAs) with different and known regio-selectivities are currently emerging as efficient tools to produce fully defined paCOS in quantities sufficient to probe their bioactivities. In this review, we describe the current state of the art on how CDAs can be used in forward and reverse mode to produce all of the possible paCOS dimers, trimers, and tetramers, most of the pentamers and many of the hexamers. In addition, we describe the biotechnological production of the required fully acetylated and fully deacetylated oligomer substrates, as well as the purification and characterisation of the paCOS products.
Keywords: CDA; chitin; chitin deacetylase; chitinase; chito-oligosaccharide; chitosan; chitosanase; paCOS; pattern of acetylation; regio-selective.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- El Gueddari N.E., Moerschbacher B.M. A bioactivity matrix for chitosans as elicitors of disease resistance reactions in wheat. Adv. Chitin Sci. 2004;VII:56–59.
-
- El Gueddari N.E., Kolkenbrock S., Schaaf A., Chilukoti N., Brunel F., Gorzelanny C., Fehser S., Chachra S., Bernard F., Nampally M., et al. Chitin and chitosan modifying enzymes: Versatile novel tools for the analysis of structure-function relationship of partially acetylaed chitosans. Adv. Chitin Sci. 2014;XIV:40–47.
-
- Moerschbacher B.M. Bio-activity matrices of chitosans in plant protection. In: Gnanamanickam S.S., Balasubramanian R., Anand N., editors. Emerging Trends in Plant-Microbe Interactions. University of Madras; Chennai, India: 2005. pp. 186–190.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

