A Novel Bead-Based Immunoassay for the Measurement of Heat Shock Proteins 27 and 70
- PMID: 33105839
- PMCID: PMC7690633
- DOI: 10.3390/pathogens9110863
A Novel Bead-Based Immunoassay for the Measurement of Heat Shock Proteins 27 and 70
Abstract
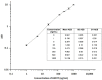
Heat shock proteins (HSPs) play an essential role in protecting proteins from denaturation and are implicated in diverse pathophysiological conditions like cardiovascular diseases, cancer, infections, and neurodegenerative diseases. Scientific evidence indicates that if HSP expression falls below a certain level, cells become sensitive to oxidative damage that accelerates protein aggregation diseases. On the other hand, persistently enhanced levels of HSP can lead to inflammatory and oncogenic changes. To date, although techniques for measuring HSPs exist, these assays are limited for use in specific sample types or are time consuming. Therefore, in the present study, we developed a single-molecule assay digital ELISA technology (Single Molecule Array-SIMOA) for the measurement of HSPs, which is time effective and can be adapted to measure multiple analytes simultaneously from a single sample. This technique combines two distinct HSP-specific antibodies that recognize different epitopes on the HSP molecule. A recombinant human HSP protein was used as the standard material. The assay performance characteristics were evaluated by repeated testing of samples spiked with HSP peptide at different levels. The limit of detection was 0.16 and 2 ng/mL for HSP27 and HSP70, respectively. The inter- and intra-assay coefficients of variation were less than 20% in all tested conditions for both HSPs. The HSP levels assayed after serial dilution of samples portrayed dilutional linearity (on average 109%, R2 = 0.998, p < 0.001, for HSP27 and 93%, R2 = 0.994, p < 0.001, for HSP70). A high linear response was also demonstrated with admixtures of plasma exhibiting relatively very low and high levels of HSP70 (R2 = 0.982, p < 0.001). Analyte spike recovery varied between 57% and 95%. Moreover, the relative HSP values obtained using Western blotting correlated significantly with HSP values obtained with the newly developed SIMOA assay (r = 0.815, p < 0.001 and r = 0,895, p < 0.001 for HSP70 and HSP27, respectively), indicating that our method is reliable. In conclusion, the assay demonstrates analytical performance for the accurate assessment of HSPs in various sample types and offers the advantage of a huge range of dilution linearity, indicating that samples with HSP concentration highly above the calibration range can be diluted into range without affecting the precision of the assay.
Keywords: cell lysates; heat shock proteins; plasma; single molecule assay.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





Similar articles
-
Determination of intracellular heat shock protein 70 using a newly developed cell lysate immunometric assay.J Immunol Methods. 2003 Mar 1;274(1-2):271-9. doi: 10.1016/s0022-1759(03)00004-8. J Immunol Methods. 2003. PMID: 12609553
-
Heat shock protein expression independently predicts clinical outcome in prostate cancer.Cancer Res. 2000 Dec 15;60(24):7099-105. Cancer Res. 2000. PMID: 11156417
-
Expression of heat shock proteins in medulloblastoma.J Neurosurg Pediatr. 2013 Nov;12(5):452-7. doi: 10.3171/2013.7.PEDS1376. Epub 2013 Aug 30. J Neurosurg Pediatr. 2013. PMID: 23992239
-
The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system.Int J Hyperthermia. 2005 Aug;21(5):379-92. doi: 10.1080/02656730500069955. Int J Hyperthermia. 2005. PMID: 16048836 Review.
-
Heat Shock Proteins: Therapeutic Perspectives in Inflammatory Disorders.Recent Pat Inflamm Allergy Drug Discov. 2017;10(2):94-104. doi: 10.2174/1872213X10666161213163301. Recent Pat Inflamm Allergy Drug Discov. 2017. PMID: 27978789 Review.
Cited by
-
A systematic review of approaches to assess fish health responses to anthropogenic threats in freshwater ecosystems.Conserv Physiol. 2024 May 4;12(1):coae022. doi: 10.1093/conphys/coae022. eCollection 2024. Conserv Physiol. 2024. PMID: 38706739 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

