An Approach to Ring Resonator Biosensing Assisted by Dielectrophoresis: Design, Simulation and Fabrication
- PMID: 33105846
- PMCID: PMC7690605
- DOI: 10.3390/mi11110954
An Approach to Ring Resonator Biosensing Assisted by Dielectrophoresis: Design, Simulation and Fabrication
Abstract
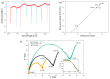
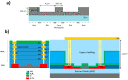
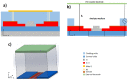
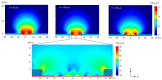
The combination of extreme miniaturization with a high sensitivity and the potential to be integrated in an array form on a chip has made silicon-based photonic microring resonators a very attractive research topic. As biosensors are approaching the nanoscale, analyte mass transfer and bonding kinetics have been ascribed as crucial factors that limit their performance. One solution may be a system that applies dielectrophoretic forces, in addition to microfluidics, to overcome the diffusion limits of conventional biosensors. Dielectrophoresis, which involves the migration of polarized dielectric particles in a non-uniform alternating electric field, has previously been successfully applied to achieve a 1000-fold improved detection efficiency in nanopore sensing and may significantly increase the sensitivity in microring resonator biosensing. In the current work, we designed microring resonators with integrated electrodes next to the sensor surface that may be used to explore the effect of dielectrophoresis. The chip design, including two different electrode configurations, electric field gradient simulations, and the fabrication process flow of a dielectrohoresis-enhanced microring resonator-based sensor, is presented in this paper. Finite element method (FEM) simulations calculated for both electrode configurations revealed ∇E2 values above 1017 V2m-3 around the sensing areas. This is comparable to electric field gradients previously reported for successful interactions with larger molecules, such as proteins and antibodies.
Keywords: biosensor; dielectrophoresis; mass transfer; micro fabrication; microring resonator; photonic sensor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Miniature microring resonator sensor based on a hybrid plasmonic waveguide.Sensors (Basel). 2011;11(7):6856-67. doi: 10.3390/s110706856. Epub 2011 Jul 1. Sensors (Basel). 2011. PMID: 22163989 Free PMC article.
-
Label-free biosensor based on an electrical tracing-assisted silicon microring resonator with a low-cost broadband source.Biosens Bioelectron. 2013 Aug 15;46:15-21. doi: 10.1016/j.bios.2013.02.002. Epub 2013 Feb 26. Biosens Bioelectron. 2013. PMID: 23500471
-
Label-free biosensing using a microring resonator integrated with poly-(dimethylsiloxane) microfluidic channels.Rev Sci Instrum. 2019 Mar;90(3):035004. doi: 10.1063/1.5074134. Rev Sci Instrum. 2019. PMID: 30927803
-
Electric field-induced effects on neuronal cell biology accompanying dielectrophoretic trapping.Adv Anat Embryol Cell Biol. 2003;173:III-IX, 1-77. doi: 10.1007/978-3-642-55469-8. Adv Anat Embryol Cell Biol. 2003. PMID: 12901336 Review.
-
Silicon Photonic Biosensors Using Label-Free Detection.Sensors (Basel). 2018 Oct 18;18(10):3519. doi: 10.3390/s18103519. Sensors (Basel). 2018. PMID: 30340405 Free PMC article. Review.
Cited by
-
Electronic selection of viable Legionella cells by a video-based, quantifiable dielectrophoresis approach.Biomed Microdevices. 2025 Jul 30;27(3):37. doi: 10.1007/s10544-025-00762-1. Biomed Microdevices. 2025. PMID: 40736590 Free PMC article.
-
Editorial for the Special Issue on Biosensors and MEMS-Based Diagnostic Applications.Micromachines (Basel). 2021 Feb 25;12(3):229. doi: 10.3390/mi12030229. Micromachines (Basel). 2021. PMID: 33668692 Free PMC article.
-
Dielectrophoresis: An Approach to Increase Sensitivity, Reduce Response Time and to Suppress Nonspecific Binding in Biosensors?Biosensors (Basel). 2022 Sep 23;12(10):784. doi: 10.3390/bios12100784. Biosensors (Basel). 2022. PMID: 36290922 Free PMC article. Review.
References
-
- Lummerstorfer T., Hoffmann H. Click Chemistry on Surfaces: 1,3-Dipolar Cycloaddition Reactions of Azide-Terminated Monolayers on Silica. J. Phys. Chem. B. 2004;108:3963–3966. doi: 10.1021/jp049601t. - DOI
-
- Henriksson A., Nishiori D., Maeda H., Miyachi M., Yamanoi Y., Nishihara H. Attachment chemistry of aromatic compounds on a Silicon(100) surface. Surf. Sci. 2018;669:140–144. doi: 10.1016/j.susc.2017.11.017. - DOI
-
- Henriksson A., Hoffmann H. Click Coupling Reactions on Flat and Nanostructured Hydrogen-Passivated Silicon Surfaces. Phys. Status Solidi. 2018;216:1800683. doi: 10.1002/pssa.201800683. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

