Voltage-Gated Calcium Channels in Nonexcitable Tissues
- PMID: 33106102
- PMCID: PMC8281591
- DOI: 10.1146/annurev-physiol-031620-091043
Voltage-Gated Calcium Channels in Nonexcitable Tissues
Abstract
The identification of a gain-of-function mutation in CACNA1C as the cause of Timothy syndrome, a rare disorder characterized by cardiac arrhythmias and syndactyly, highlighted roles for the L-type voltage-gated Ca2+ channel CaV1.2 in nonexcitable cells. Previous studies in cells and animal models had suggested that several voltage-gated Ca2+ channels (VGCCs) regulated critical signaling events in various cell types that are not expected to support action potentials, but definitive data were lacking. VGCCs occupy a special position among ion channels, uniquely able to translate membrane excitability into the cytoplasmic Ca2+ changes that underlie the cellular responses to electrical activity. Yet how these channels function in cells not firing action potentials and what the consequences of their actions are in nonexcitable cells remain critical questions. The development of new animal and cellular models and the emergence of large data sets and unbiased genome screens have added to our understanding of the unanticipated roles for VGCCs in nonexcitable cells. Here, we review current knowledge of VGCC regulation and function in nonexcitable tissues and cells, with the goal of providing a platform for continued investigation.
Keywords: Timothy syndrome; nonexcitable cells; voltage-gated Ca2+ channel.
Figures

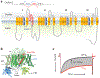

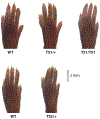

References
-
- Yue DT. 2004. The dawn of high-resolution structure for the queen of ion channels. Neuron 42:357–59 - PubMed
-
- Hille B 2001. Ion Channels of Excitable Membranes. Oxford, UK: Oxford Univ. Press
-
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, et al. 2004. CaV1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 119:19–31 - PubMed
-
- Catterall WA, Lenaeus MJ, Gamal El-Din TM. 2020. Structure and pharmacology of voltage-gated sodium and calcium channels. Annu. Rev. Pharmacol. Toxicol 60:133–54 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

