Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials
- PMID: 33106286
- PMCID: PMC7815636
- DOI: 10.1136/annrheumdis-2019-216835
Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials
Abstract
Objective: To compare the efficacy and safety of brodalumab, an interleukin-17 receptor subunit A inhibitor, with placebo, in patients with psoriatic arthritis (PsA).
Methods: Adult patients with active PsA and inadequate response to, or intolerance to, conventional treatment were enrolled into two phase III studies (NCT02029495 and NCT02024646) and randomised 1:1:1 to receive subcutaneous brodalumab 140 mg or 210 mg or placebo at weeks 0, 1 and every 2 weeks up to 24 weeks. About 30% of patients had prior use of biologics. The primary endpoint for both studies was the American College of Rheumatology 20 (ACR20) response at week 16.
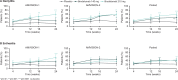
Results: 962 patients were randomised across the studies prior to early termination due to sponsor decision. The primary endpoint was met in both studies. Based on comparable design and eligibility criteria, data from both studies were pooled. Significantly more patients achieved ACR20 at week 16 in both brodalumab treatment groups (45.8% and 47.9% for 140 mg and 210 mg, respectively) versus placebo (20.9%) (p<0.0001). Similar results were observed at week 24. Significantly higher proportions of patients receiving brodalumab achieved ACR50/70, Psoriasis Area and Severity Index 75/90/100 and resolution of dactylitis and enthesitis versus placebo (p<0.01). Adverse event rates were similar across treatments at week 16 (54.4%, 51.6% and 54.5% for placebo, brodalumab 140 mg and 210 mg, respectively). No new safety signals were reported.
Conclusion: Brodalumab was associated with rapid and significant improvements in signs and symptoms of PsA versus placebo. Brodalumab was well tolerated, with a safety profile consistent with other interleukin-17 inhibitors.
Keywords: DMARDs (biologic); autoimmune diseases; psoriatic arthritis.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PJM reports, outside the submitted work, grants and personal fees from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Janssen, Eli Lilly, Novartis, SUN Pharma, UCB Pharma; personal fees from Genentech, Boehringer Ingelheim, Galapagos, Gilead and GlaxoSmithKline. PSH reports, outside the submitted work, grants, personal fees and non-financial support from AbbVie; grants from Amgen, Celgene, Janssen, MSD, Pfizer and UCB; and personal fees from Galapagos. KFH was an employee of LEO Pharma at the time this study was conducted. KFH also reports, outside the submitted work, grants from AbbVie, Celgene, LEO Pharma and Novartis; personal fees from AbbVie, CSL Behring, Eli Lilly, Janssen, LEO Pharma, Novartis and Pfizer. KR is an employee of LEO Pharma. IBM reports personal fees from LEO Pharma during the conduct of the study. IBM also reports, outside the submitted work, grants and personal fees from Celgene, Compugen and UCB; grants from AstraZeneca, Novartis and Roche; personal fees from AbbVie, Galvani, Eli Lilly and Pfizer.
Figures


References
-
- Sokoll KB, Helliwell PS. Comparison of disability and quality of life in rheumatoid and psoriatic arthritis. J Rheumatol 2001;28:1842–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

