Plasmodium DEH is ER-localized and crucial for oocyst mitotic division during malaria transmission
- PMID: 33106323
- PMCID: PMC7652392
- DOI: 10.26508/lsa.202000879
Plasmodium DEH is ER-localized and crucial for oocyst mitotic division during malaria transmission
Abstract
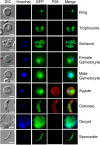
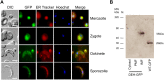
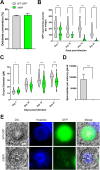
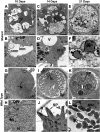
Cells use fatty acids (FAs) for membrane biosynthesis, energy storage, and the generation of signaling molecules. 3-hydroxyacyl-CoA dehydratase-DEH-is a key component of very long chain fatty acid synthesis. Here, we further characterized in-depth the location and function of DEH, applying in silico analysis, live cell imaging, reverse genetics, and ultrastructure analysis using the mouse malaria model Plasmodium berghei DEH is evolutionarily conserved across eukaryotes, with a single DEH in Plasmodium spp. and up to three orthologs in the other eukaryotes studied. DEH-GFP live-cell imaging showed strong GFP fluorescence throughout the life-cycle, with areas of localized expression in the cytoplasm and a circular ring pattern around the nucleus that colocalized with ER markers. Δdeh mutants showed a small but significant reduction in oocyst size compared with WT controls from day 10 postinfection onwards, and endomitotic cell division and sporogony were completely ablated, blocking parasite transmission from mosquito to vertebrate host. Ultrastructure analysis confirmed degeneration of Δdeh oocysts, and a complete lack of sporozoite budding. Overall, DEH is evolutionarily conserved, localizes to the ER, and plays a crucial role in sporogony.
© 2020 Guttery et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Plasmodium P-Type Cyclin CYC3 Modulates Endomitotic Growth during Oocyst Development in Mosquitoes.PLoS Pathog. 2015 Nov 13;11(11):e1005273. doi: 10.1371/journal.ppat.1005273. eCollection 2015 Nov. PLoS Pathog. 2015. PMID: 26565797 Free PMC article.
-
Dysregulated gene expression in oocysts of Plasmodium berghei LAP mutants.Mol Biochem Parasitol. 2019 Apr;229:1-5. doi: 10.1016/j.molbiopara.2019.02.001. Epub 2019 Feb 10. Mol Biochem Parasitol. 2019. PMID: 30753856 Free PMC article.
-
Plasmodium berghei Cap93, a novel oocyst capsule-associated protein, plays a role in sporozoite development.Parasit Vectors. 2017 Aug 25;10(1):399. doi: 10.1186/s13071-017-2337-8. Parasit Vectors. 2017. PMID: 28841886 Free PMC article.
-
Plasmodium Cysteine Repeat Modular Proteins 3 and 4 are essential for malaria parasite transmission from the mosquito to the host.Malar J. 2011 Mar 31;10:71. doi: 10.1186/1475-2875-10-71. Malar J. 2011. PMID: 21453484 Free PMC article.
-
Type II fatty acid biosynthesis is essential for Plasmodium falciparum sporozoite development in the midgut of Anopheles mosquitoes.Eukaryot Cell. 2014 May;13(5):550-9. doi: 10.1128/EC.00264-13. Epub 2013 Dec 2. Eukaryot Cell. 2014. PMID: 24297444 Free PMC article.
Cited by
-
A CRISPR homing screen finds a chloroquine resistance transporter-like protein of the Plasmodium oocyst essential for mosquito transmission of malaria.Nat Commun. 2025 Apr 24;16(1):3895. doi: 10.1038/s41467-025-59099-1. Nat Commun. 2025. PMID: 40274854 Free PMC article.
-
Plasmodium berghei oocysts possess fatty acid synthesis and scavenging routes.Sci Rep. 2023 Aug 5;13(1):12700. doi: 10.1038/s41598-023-39708-z. Sci Rep. 2023. PMID: 37543672 Free PMC article.
-
Zinc supplementation modifies brain tissue transcriptome of Apis mellifera honeybees.BMC Genomics. 2022 Apr 8;23(1):282. doi: 10.1186/s12864-022-08464-1. BMC Genomics. 2022. PMID: 35395723 Free PMC article.
-
Chemical Biology Tools To Investigate Malaria Parasites.Chembiochem. 2021 Jul 1;22(13):2219-2236. doi: 10.1002/cbic.202000882. Epub 2021 Mar 31. Chembiochem. 2021. PMID: 33570245 Free PMC article. Review.
-
A 3D culture model facilitates mass production of in vitro Plasmodium falciparum haemolymph-like sporozoites.Malar J. 2025 Jul 10;24(1):224. doi: 10.1186/s12936-025-05471-x. Malar J. 2025. PMID: 40640876 Free PMC article.
References
-
- Bach L, Michaelson LV, Haslam R, Bellec Y, Gissot L, Marion J, Da Costa M, Boutin JP, Miquel M, Tellier F, et al. (2008) The very-long-chain hydroxy fatty acyl-CoA dehydratase PASTICCINO2 is essential and limiting for plant development. Proc Natl Acad Sci U S A 105: 14727–14731. 10.1073/pnas.0805089105 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
