Over-activation of primate subgenual cingulate cortex enhances the cardiovascular, behavioral and neural responses to threat
- PMID: 33106488
- PMCID: PMC7588412
- DOI: 10.1038/s41467-020-19167-0
Over-activation of primate subgenual cingulate cortex enhances the cardiovascular, behavioral and neural responses to threat
Abstract
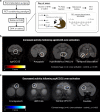
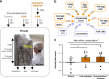
Stress-related disorders such as depression and anxiety are characterized by enhanced negative emotion and physiological dysfunction. Whilst elevated activity within area 25 of the subgenual anterior cingulate cortex (sgACC/25) has been implicated in these illnesses, it is unknown whether this over-activity is causal. By combining targeted intracerebral microinfusions with cardiovascular and behavioral monitoring in marmosets, we show that over-activation of sgACC/25 reduces vagal tone and heart rate variability, alters cortisol dynamics during stress and heightens reactivity to proximal and distal threat. 18F-FDG PET imaging shows these changes are accompanied by altered activity within a network of brain regions including the amygdala, hypothalamus and dorsolateral prefrontal cortex. Ketamine, shown to have rapid antidepressant effects, fails to reverse elevated arousal to distal threat contrary to the beneficial effects we have previously demonstrated on over-activation induced reward blunting, illustrating the symptom-specificity of its actions.
Conflict of interest statement
The authors declare no competing interests.
Figures




Comment in
-
Heightening the threat.Nat Rev Neurosci. 2021 Jan;22(1):4-5. doi: 10.1038/s41583-020-00417-5. Nat Rev Neurosci. 2021. PMID: 33262476 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

