Presence of low virulence chytrid fungi could protect European amphibians from more deadly strains
- PMID: 33106491
- PMCID: PMC7589487
- DOI: 10.1038/s41467-020-19241-7
Presence of low virulence chytrid fungi could protect European amphibians from more deadly strains
Abstract
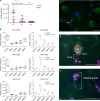
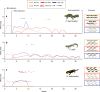
Wildlife diseases are contributing to the current Earth's sixth mass extinction; one disease, chytridiomycosis, has caused mass amphibian die-offs. While global spread of a hypervirulent lineage of the fungus Batrachochytrium dendrobatidis (BdGPL) causes unprecedented loss of vertebrate diversity by decimating amphibian populations, its impact on amphibian communities is highly variable across regions. Here, we combine field data with in vitro and in vivo trials that demonstrate the presence of a markedly diverse variety of low virulence isolates of BdGPL in northern European amphibian communities. Pre-exposure to some of these low virulence isolates protects against disease following subsequent exposure to highly virulent BdGPL in midwife toads (Alytes obstetricans) and alters infection dynamics of its sister species B. salamandrivorans in newts (Triturus marmoratus), but not in salamanders (Salamandra salamandra). The key role of pathogen virulence in the complex host-pathogen-environment interaction supports efforts to limit pathogen pollution in a globalized world.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Berger L, et al. History and recent progress on chytridiomycosis in amphibians. Fungal Ecol. 2016;19:89–99. doi: 10.1016/j.funeco.2015.09.007. - DOI

