T cell-intrinsic role for Nod2 in protection against Th17-mediated uveitis
- PMID: 33106495
- PMCID: PMC7589501
- DOI: 10.1038/s41467-020-18961-0
T cell-intrinsic role for Nod2 in protection against Th17-mediated uveitis
Abstract
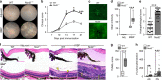
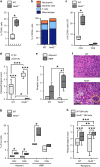
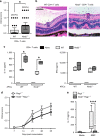
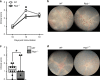
Mutations in nucleotide-binding oligomerization domain-containing protein 2 (NOD2) cause Blau syndrome, an inflammatory disorder characterized by uveitis. The antimicrobial functions of Nod2 are well-established, yet the cellular mechanisms by which dysregulated Nod2 causes uveitis remain unknown. Here, we report a non-conventional, T cell-intrinsic function for Nod2 in suppression of Th17 immunity and experimental uveitis. Reconstitution of lymphopenic hosts with Nod2-/- CD4+ T cells or retina-specific autoreactive CD4+ T cells lacking Nod2 reveals a T cell-autonomous, Rip2-independent mechanism for Nod2 in uveitis. In naive animals, Nod2 operates downstream of TCR ligation to suppress activation of memory CD4+ T cells that associate with an autoreactive-like profile involving IL-17 and Ccr7. Interestingly, CD4+ T cells from two Blau syndrome patients show elevated IL-17 and increased CCR7. Our data define Nod2 as a T cell-intrinsic rheostat of Th17 immunity, and open new avenues for T cell-based therapies for Nod2-associated disorders such as Blau syndrome.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Sarens, I. L. et al. Blau syndrome-associated uveitis: preliminary results from an international prospective interventional case series. Am. J. Ophthalmol.187, 158–166 (2017). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

