Cancer therapy shapes the fitness landscape of clonal hematopoiesis
- PMID: 33106634
- PMCID: PMC7891089
- DOI: 10.1038/s41588-020-00710-0
Cancer therapy shapes the fitness landscape of clonal hematopoiesis
Abstract
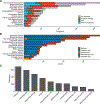
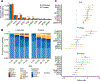
Acquired mutations are pervasive across normal tissues. However, understanding of the processes that drive transformation of certain clones to cancer is limited. Here we study this phenomenon in the context of clonal hematopoiesis (CH) and the development of therapy-related myeloid neoplasms (tMNs). We find that mutations are selected differentially based on exposures. Mutations in ASXL1 are enriched in current or former smokers, whereas cancer therapy with radiation, platinum and topoisomerase II inhibitors preferentially selects for mutations in DNA damage response genes (TP53, PPM1D, CHEK2). Sequential sampling provides definitive evidence that DNA damage response clones outcompete other clones when exposed to certain therapies. Among cases in which CH was previously detected, the CH mutation was present at tMN diagnosis. We identify the molecular characteristics of CH that increase risk of tMN. The increasing implementation of clinical sequencing at diagnosis provides an opportunity to identify patients at risk of tMN for prevention strategies.
Conflict of interest statement
COMPETING INTEREST DECLARATION
The remaining authors declare no competing interests.
Figures




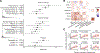

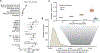
Comment in
-
Cancer Treatment Exerts Selective Pressure on Premalignant Clones.Cancer Discov. 2021 Jan;11(1):9. doi: 10.1158/2159-8290.CD-RW2020-162. Epub 2020 Nov 6. Cancer Discov. 2021. PMID: 33158847
References
-
- Sabarinathan R et al. The whole-genome panorama of cancer drivers. doi:10.1101/190330. - DOI
METHODS-ONLY REFERENCES
-
- Papaemmanuil E et al. Identification of Novel Somatic Mutations in SF3B1, a Gene Encoding a Core Component of RNA Splicing Machinery, in Myelodysplasia with Ring Sideroblasts and Other Common Cancers. European Journal of Cancer vol. 47 7 (2011).
-
- Campo E et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. (IARC Who Classification of Tum, 2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

