A new neutrophil subset promotes CNS neuron survival and axon regeneration
- PMID: 33106668
- PMCID: PMC7677206
- DOI: 10.1038/s41590-020-00813-0
A new neutrophil subset promotes CNS neuron survival and axon regeneration
Abstract
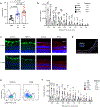
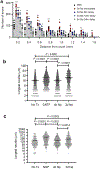
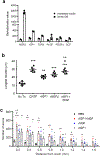
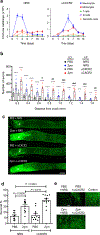
Transected axons typically fail to regenerate in the central nervous system (CNS), resulting in chronic neurological disability in individuals with traumatic brain or spinal cord injury, glaucoma and ischemia-reperfusion injury of the eye. Although neuroinflammation is often depicted as detrimental, there is growing evidence that alternatively activated, reparative leukocyte subsets and their products can be deployed to improve neurological outcomes. In the current study, we identify a unique granulocyte subset, with characteristics of an immature neutrophil, that had neuroprotective properties and drove CNS axon regeneration in vivo, in part via secretion of a cocktail of growth factors. This pro-regenerative neutrophil promoted repair in the optic nerve and spinal cord, demonstrating its relevance across CNS compartments and neuronal populations. Our findings could ultimately lead to the development of new immunotherapies that reverse CNS damage and restore lost neurological function across a spectrum of diseases.
Conflict of interest statement
Competing Interests statement
The authors have no competing interests.
Figures







Comment in
-
A new cell subtype that confers neuroprotection.Nat Immunol. 2020 Dec;21(12):1477-1478. doi: 10.1038/s41590-020-00821-0. Nat Immunol. 2020. PMID: 33139916 No abstract available.
-
Neurorestorative neutrophils.Nat Rev Immunol. 2021 Jan;21(1):2-3. doi: 10.1038/s41577-020-00485-9. Nat Rev Immunol. 2021. PMID: 33257820 No abstract available.
-
Revealing the diversity of neutrophil functions and subsets.Cell Mol Immunol. 2021 Apr;18(4):781-783. doi: 10.1038/s41423-021-00638-3. Epub 2021 Jan 29. Cell Mol Immunol. 2021. PMID: 33514848 Free PMC article. No abstract available.
-
Bridging the gap-Immune cells that can repair nerves.Cell Mol Immunol. 2021 Apr;18(4):784-786. doi: 10.1038/s41423-021-00642-7. Epub 2021 Feb 12. Cell Mol Immunol. 2021. PMID: 33580168 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

