Nutritional modulation of heart failure in mitochondrial pyruvate carrier-deficient mice
- PMID: 33106690
- PMCID: PMC7957960
- DOI: 10.1038/s42255-020-00296-1
Nutritional modulation of heart failure in mitochondrial pyruvate carrier-deficient mice
Abstract
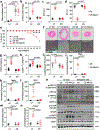
The myocardium is metabolically flexible; however, impaired flexibility is associated with cardiac dysfunction in conditions including diabetes and heart failure. The mitochondrial pyruvate carrier (MPC) complex, composed of MPC1 and MPC2, is required for pyruvate import into the mitochondria. Here we show that MPC1 and MPC2 expression is downregulated in failing human and mouse hearts. Mice with cardiac-specific deletion of Mpc2 (CS-MPC2-/-) exhibited normal cardiac size and function at 6 weeks old, but progressively developed cardiac dilation and contractile dysfunction, which was completely reversed by a high-fat, low-carbohydrate ketogenic diet. Diets with higher fat content, but enough carbohydrate to limit ketosis, also improved heart failure, while direct ketone body provisioning provided only minor improvements in cardiac remodelling in CS-MPC2-/- mice. An acute fast also improved cardiac remodelling. Together, our results reveal a critical role for mitochondrial pyruvate use in cardiac function, and highlight the potential of dietary interventions to enhance cardiac fat metabolism to prevent or reverse cardiac dysfunction and remodelling in the setting of MPC deficiency.
Conflict of interest statement
COMPETING INTERESTS
KSM previously received research support from Cirius Therapeutics, and BNF is a stockholder and scientific advisory board member of Cirius Therapeutics. RLV held patents on the synthesis and uses of ketone esters, and MTK is a co-inventor in the synthesis of ketone esters. All other authors have declared that no conflict of interest exists.
Figures







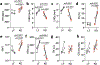







References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL128349/HL/NHLBI NIH HHS/United States
- R01 DK126622/DK/NIDDK NIH HHS/United States
- R00 HL136658/HL/NHLBI NIH HHS/United States
- K99 HL136658/HL/NHLBI NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- R21 AT010520/AT/NCCIH NIH HHS/United States
- R01 HL133178/HL/NHLBI NIH HHS/United States
- R42 DK121652/DK/NIDDK NIH HHS/United States
- R01 DK089312/DK/NIDDK NIH HHS/United States
- R01 HL119225/HL/NHLBI NIH HHS/United States
- R01 HL147968/HL/NHLBI NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- S10 OD028597/OD/NIH HHS/United States
- R01 DK104735/DK/NIDDK NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- R56 DK115764/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

