Probing infectious disease by single-cell RNA sequencing: Progresses and perspectives
- PMID: 33106757
- PMCID: PMC7577221
- DOI: 10.1016/j.csbj.2020.10.016
Probing infectious disease by single-cell RNA sequencing: Progresses and perspectives
Abstract
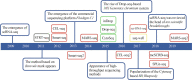
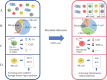
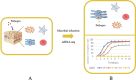
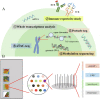
The increasing application of single-cell RNA sequencing (scRNA-seq) technology in life science and biomedical research has significantly increased our understanding of the cellular heterogeneities in immunology, oncology and developmental biology. This review will summarize the development of various scRNA-seq technologies; primarily discussing the application of scRNA-seq on infectious diseases, and exploring the current development, challenges, and potential applications of scRNA-seq technology in the future.
Keywords: 3C, Chromosome Conformation Capture; ACE2, Angiotensin-Converting Enzyme 2; ARDS, acute respiratory distress syndrome; ATAC-seq, Assay for Transposase-Accessible Chromatin using sequencing; BCR, B cell receptor; CEL-seq, Cell Expression by Linear amplification and Sequencing; CLU, clusterin; COVID-19, corona virus disease 2019; CRISPR, Clustered Regularly Interspaced Short Palindromic Repeats; CytoSeq, gene expression cytometry; DENV, dengue virus; FACS, fluorescence-activated cell sorting; GNLY, granulysin; GO analysis, Gene Ontology analysis; HIV, Human Immunodeficiency Virus; IAV, Influenza A virus; IGHV/HD/HJ/HC, Immune globulin heavy V/D/J/C/ region; IGLV/LJ/LC, Immune globulin light V/J/C/ region; ILC, Innate Lymphoid Cell; Infectious diseases; LIGER, Linked Inference of Genomics Experimental Relationships; MAGIC, Markov Affinity-based Graph Imputation of Cells; MARS-seq, Massively parallel single-cell RNA sequencing; MATCHER, Manifold Alignment To CHaracterize Experimental Relationships; MCMV, mouse cytomegalovirus; MERFISH, Multiplexed, Error Robust Fluorescent In Situ Hybridization; MLV, Moloney Murine Leukemia Virus; MOFA, Multi-Omics Factor Analysis; MOI, multiplicity of infection; PBMCs, peripheral blood mononuclear cells; PLAC8, placenta-associated 8; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SAVER, Single-cell Analysis Via Expression Recovery; SPLit-seq, split pool ligation-based tranome sequencing; STARTRAC, Single T-cell Analysis by RNA sequencing and TCR TRACking; STRT-seq, Single-cell Tagged Reverse Transcription sequencing; Single-cell RNA sequencing; TCR, T cell receptor; TSLP, thymic stromal lymphopoietin; UMAP, Uniform Manifold Approximation and Projection; UMI, Unique Molecular Identifier; mcSCRB-seq, molecular crowding single-cell RNA barcoding and sequencing; pDCs, plasmacytoid dendritic cells; scRNA-seq, single cell RNA sequencing technology; sci-RNA-seq, single-cell combinatorial indexing RNA sequencing; seqFISH, sequential Fluorescent In Situ Hybridization; smart-seq, switching mechanism at 5′ end of the RNA transcript sequencing; t-SNE, t-Distributed stochastic neighbor embedding.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Computational solutions for spatial transcriptomics.Comput Struct Biotechnol J. 2022 Sep 1;20:4870-4884. doi: 10.1016/j.csbj.2022.08.043. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36147664 Free PMC article. Review.
-
Unambiguous detection of SARS-CoV-2 subgenomic mRNAs with single-cell RNA sequencing.Microbiol Spectr. 2023 Sep 7;11(5):e0077623. doi: 10.1128/spectrum.00776-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 37676044 Free PMC article.
-
Enriching and Characterizing T Cell Repertoires from 3' Barcoded Single-Cell Whole Transcriptome Amplification Products.Methods Mol Biol. 2022;2574:159-182. doi: 10.1007/978-1-0716-2712-9_7. Methods Mol Biol. 2022. PMID: 36087201 Free PMC article.
-
Hydrop enables droplet-based single-cell ATAC-seq and single-cell RNA-seq using dissolvable hydrogel beads.Elife. 2022 Feb 23;11:e73971. doi: 10.7554/eLife.73971. Elife. 2022. PMID: 35195064 Free PMC article.
-
Application of single-cell RNA sequencing technology in liver diseases: a narrative review.Ann Transl Med. 2021 Oct;9(20):1598. doi: 10.21037/atm-21-4824. Ann Transl Med. 2021. PMID: 34790804 Free PMC article. Review.
Cited by
-
Development of Single-Cell Transcriptomics and Its Application in COVID-19.Viruses. 2022 Oct 16;14(10):2271. doi: 10.3390/v14102271. Viruses. 2022. PMID: 36298825 Free PMC article. Review.
-
Bibliometric review of ATAC-Seq and its application in gene expression.Brief Bioinform. 2022 May 13;23(3):bbac061. doi: 10.1093/bib/bbac061. Brief Bioinform. 2022. PMID: 35255493 Free PMC article. Review.
-
Artificial Intelligence in Differential Diagnostics of Meningitis: A Nationwide Study.Diagnostics (Basel). 2021 Mar 28;11(4):602. doi: 10.3390/diagnostics11040602. Diagnostics (Basel). 2021. PMID: 33800653 Free PMC article.
-
Analysis of the Immune Responses in the Ileum of Gnotobiotic Pigs Infected with the Recombinant GII.p12_GII.3 Human Norovirus by mRNA Sequencing.Viruses. 2021 Jan 11;13(1):92. doi: 10.3390/v13010092. Viruses. 2021. PMID: 33440894 Free PMC article.
-
Advancements in technology for characterizing the tumor immune microenvironment.Int J Biol Sci. 2024 Mar 25;20(6):2151-2167. doi: 10.7150/ijbs.92525. eCollection 2024. Int J Biol Sci. 2024. PMID: 38617534 Free PMC article. Review.
References
-
- Clark S.J., Smallwood S.A., Lee H.J., Krueger F., Reik W., Kelsey G. Genome-wide base-resolution mapping of DNA methylation in single cells using single-cell bisulfite sequencing (scBS-seq) Nat Protoc. 2017;12:534–547. - PubMed
-
- Corces MR, Granja JM, Shams S, Louie BH, Seoane JA, Zhou W, Silva TC, Groeneveld C, Wong CK, Cho SW, Satpathy AT, Mumbach MR, Hoadley KA, Robertson AG, Sheffield NC, Felau I, Castro MAA, Berman BP, Staudt LM, Zenklusen JC, Laird PW, Curtis C, Cancer Genome Atlas Analysis N, Greenleaf WJ, Chang HY. The chromatin accessibility landscape of primary human cancers. Science 2018;362. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
