This is a preprint.
COVID-19 neutralizing antibodies predict disease severity and survival
- PMID: 33106822
- PMCID: PMC7587842
- DOI: 10.1101/2020.10.15.20213512
COVID-19 neutralizing antibodies predict disease severity and survival
Update in
-
COVID-19-neutralizing antibodies predict disease severity and survival.Cell. 2021 Jan 21;184(2):476-488.e11. doi: 10.1016/j.cell.2020.12.015. Epub 2020 Dec 15. Cell. 2021. PMID: 33412089 Free PMC article.
Abstract
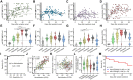
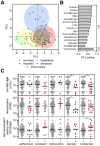
COVID-19 exhibits variable symptom severity ranging from asymptomatic to life-threatening, yet the relationship between severity and the humoral immune response is poorly understood. We examined antibody responses in 113 COVID-19 patients and found that severe cases resulting in intubation or death exhibited increased inflammatory markers, lymphopenia, and high anti-RBD antibody levels. While anti-RBD IgG levels generally correlated with neutralization titer, quantitation of neutralization potency revealed that high potency was a predictor of survival. In addition to neutralization of wild-type SARS-CoV-2, patient sera were also able to neutralize the recently emerged SARS-CoV-2 mutant D614G, suggesting protection from reinfection by this strain. However, SARS-CoV-2 sera was unable to cross-neutralize a highly-homologous pre-emergent bat coronavirus, WIV1-CoV, that has not yet crossed the species barrier. These results highlight the importance of neutralizing humoral immunity on disease progression and the need to develop broadly protective interventions to prevent future coronavirus pandemics.
Figures
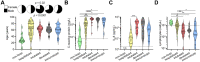


References
-
- Arvin A. M. et al. (2020) ‘A perspective on potential antibody-dependent enhancement of SARS-CoV-2’, Nature, 584(7821), pp. 353–363. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
