Safety and efficacy of sucroferric oxyhydroxide in pediatric patients with chronic kidney disease
- PMID: 33106892
- PMCID: PMC8009783
- DOI: 10.1007/s00467-020-04805-y
Safety and efficacy of sucroferric oxyhydroxide in pediatric patients with chronic kidney disease
Abstract
Background: Pediatric patients with advanced chronic kidney disease (CKD) are often prescribed oral phosphate binders (PBs) for the management of hyperphosphatemia. However, available PBs have limitations, including unfavorable tolerability and safety.
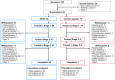
Methods: This phase 3, multicenter, randomized, open-label study investigated safety and efficacy of sucroferric oxyhydroxide (SFOH) in pediatric and adolescent subjects with CKD and hyperphosphatemia. Subjects were randomized to SFOH or calcium acetate (CaAc) for a 10-week dose titration (stage 1), followed by a 24-week safety extension (stage 2). Primary efficacy endpoint was change in serum phosphorus from baseline to the end of stage 1 in the SFOH group. Safety endpoints included treatment-emergent adverse events (TEAEs).
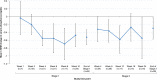
Results: Eighty-five subjects (2-18 years) were randomized and treated (SFOH, n = 66; CaAc, n = 19). Serum phosphorus reduction from baseline to the end of stage 1 in the overall SFOH group (least squares [LS] mean ± standard error [SE]) was - 0.488 ± 0.186 mg/dL; p = 0.011 (post hoc analysis). Significant reductions in serum phosphorus were observed in subjects aged ≥ 12 to ≤ 18 years (LS mean ± SE - 0.460 ± 0.195 mg/dL; p = 0.024) and subjects with serum phosphorus above age-related normal ranges at baseline (LS mean ± SE - 0.942 ± 0.246 mg/dL; p = 0.005). Similar proportions of subjects reported ≥ 1 TEAE in the SFOH (75.8%) and CaAc (73.7%) groups. Withdrawal due to TEAEs was more common with CaAc (31.6%) than with SFOH (18.2%).
Conclusions: SFOH effectively managed serum phosphorus in pediatric patients with a low pill burden and a safety profile consistent with that reported in adult patients.
Keywords: Children; Chronic kidney disease; Hyperphosphatemia; Phosphate binder; Safety profile; Sucroferric oxyhydroxide.
Conflict of interest statement
Larry A. Greenbaum and Günter Klaus have served as consultants for Vifor Pharmaceuticals. Larysa Wickman has served as a consultant for Novartis Pharma. Amandine Perrin and Milica Enoiu are employees of Vifor Pharma.
Figures

References
-
- Kidney Disease: Improving Global Outcomes CKD-MBD Working Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl:S1–S130 - PubMed
-
- Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

