Exploring the Use of Oral Pre-exposure Prophylaxis (PrEP) Among Women from Durban, South Africa as Part of the HIV Prevention Package in a Clinical Trial
- PMID: 33106996
- PMCID: PMC7973915
- DOI: 10.1007/s10461-020-03072-0
Exploring the Use of Oral Pre-exposure Prophylaxis (PrEP) Among Women from Durban, South Africa as Part of the HIV Prevention Package in a Clinical Trial
Abstract
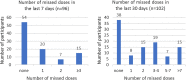
HIV endpoint-driven clinical trials in Africa enroll women who are at heightened risk of acquiring HIV. In 2017, the South African Medical Research Council recommended the provision of oral pre-exposure prophylaxis (PrEP) in HIV prevention trials, at which time the Evidence for Contraceptive Options and HIV Outcomes trial was ongoing and began to provide PrEP on-site at some trial sites. We interviewed 132 women who initiated PrEP on-site at the Durban, South Africa trial site to explore PrEP use, and conducted phone-based interviews 4-6 months post-trial exit to explore post-trial PrEP access. PrEP uptake was high (42.6%). Among women initiating PrEP on-site, 87.9% felt at risk of acquiring HIV. Most women (> 90%) heard of PrEP for the first time from study staff and three-quarters who initiated PrEP on-site continued at trial-exit. PrEP use declined post-trial exit with more than 50% of women discontinuing PrEP, and barriers relating to access emerged.
Keywords: Clinical trials; HIV prevention; Oral pre-exposure prophylaxis; Women.
Conflict of interest statement
The authors declares that they have no conflict of interest.
Figures
References
-
- Avert. HIV and AIDS in South Africa. https://www.avert.org/professionals/hiv-around-world/sub-saharan-africa/... (2020). Accessed 2 Mar 2020.
-
- UNAIDS. Country: South Africa. https://www.unaids.org/en/regionscountries/countries/southafrica (2020). Accessed 2 Mar 2020.
-
- World Health Organization (WHO). Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. https://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ (2015). Accessed 2 Mar 2020. - PubMed
-
- South Africa National Department of Health. Guidelines for expanding combination prevention and treatment options for sex workers: oral pre-exposure prophylaxis (PrEP) and test and treat (T&T). https://www.nicd.ac.za/assets/files/PrEP and TT guidelines—final draft—11 May 2016.pdf (2020). Accessed 2 Mar 2020.
-
- Pillay Y. Challenges of South Africa’s sex worker PrEP programme: lessons learned, moving towards to other key populations. In: 22nd International AIDS Conference Amsterdam, The Netherlands; 2018
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous