Factors Influencing Implementation of a Colorectal Cancer Screening Improvement Program in Community Health Centers: an Applied Use of Configurational Comparative Methods
- PMID: 33107003
- PMCID: PMC7652967
- DOI: 10.1007/s11606-020-06186-2
Factors Influencing Implementation of a Colorectal Cancer Screening Improvement Program in Community Health Centers: an Applied Use of Configurational Comparative Methods
Abstract
Background: Evidence-based programs such as mailed fecal immunochemical test (FIT) outreach can only affect health outcomes if they can be successfully implemented. However, attempts to implement programs are often limited by organizational-level factors.
Objectives: As part of the Strategies and Opportunities to Stop Colon Cancer in Priority Populations (STOP CRC) pragmatic trial, we evaluated how organizational factors impacted the extent to which health centers implemented a mailed FIT outreach program.
Design: Eight health centers participated in STOP CRC. The intervention consisted of customized electronic health record tools and clinical staff training to facilitate mailing of an introduction letter, FIT kit, and reminder letter. Health centers had flexibility in how they delivered the program.
Main measures: We categorized the health centers' level of implementation based on the proportion of eligible patients who were mailed a FIT kit, and applied configurational comparative methods to identify combinations of relevant organizational-level and program-level factors that distinguished among high, medium, and low implementing health centers. The factors were categorized according to the Consolidated Framework for Implementation Research model.
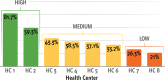
Key results: FIT tests were mailed to 21.0-81.7% of eligible participants at each health center. We identified a two-factor solution that distinguished among levels of implementation with 100% consistency and 100% coverage. The factors were having a centralized implementation team (inner setting) and mailing the introduction letter in advance of the FIT kit (intervention characteristics). Health centers with high levels of implementation had the joint presence of both factors. In health centers with medium levels of implementation, only one factor was present. Health centers with low levels of implementation had neither factor present.
Conclusions: Full implementation of the STOP CRC intervention relied on a centralized implementation team with dedicated staffing time, and the advance mailing of an introduction letter.
Trial registration: ClinicalTrials.gov Identifier: NCT01742065 Registered 05 December 2012-Prospectively registered.
Keywords: Consolidated Framework for Implementation Research; FIT tests; colorectal cancer; configurational comparative methods; fecal immunochemical tests; implementation; screening.
Conflict of interest statement
The authors declare that they do not have a conflict of interest.
Dr. Coronado: From November 2014 to August 2015, Dr. Coronado served as a co-investigator on an industry-funded study to evaluate patient adherence to an experimental blood test for colorectal cancer. The study was funded by EpiGenomics. From September 2017 to June 2018, Dr. Coronado served as the Principal Investigator on an industry-funded study to compare the clinical performance of an experimental FIT to an FDA-approved FIT. This study is funded by Quidel Corporation. Dr. Coronado has served as a scientific advisor for Exact Sciences and Guardant Health. All other authors declare no conflicts of interest.
Figures
Similar articles
-
Effectiveness of a Mailed Colorectal Cancer Screening Outreach Program in Community Health Clinics: The STOP CRC Cluster Randomized Clinical Trial.JAMA Intern Med. 2018 Sep 1;178(9):1174-1181. doi: 10.1001/jamainternmed.2018.3629. JAMA Intern Med. 2018. PMID: 30083752 Free PMC article. Clinical Trial.
-
Effect of Patient Portal Messaging Before Mailing Fecal Immunochemical Test Kit on Colorectal Cancer Screening Rates: A Randomized Clinical Trial.JAMA Netw Open. 2022 Feb 1;5(2):e2146863. doi: 10.1001/jamanetworkopen.2021.46863. JAMA Netw Open. 2022. PMID: 35119462 Free PMC article. Clinical Trial.
-
Direct-to-member mailed colorectal cancer screening outreach for Medicaid and Medicare enrollees: Implementation and effectiveness outcomes from the BeneFIT study.Cancer. 2020 Feb 1;126(3):540-548. doi: 10.1002/cncr.32567. Epub 2019 Oct 28. Cancer. 2020. PMID: 31658375 Free PMC article.
-
Moderators of the effectiveness of an intervention to increase colorectal cancer screening through mailed fecal immunochemical test kits: results from a pragmatic randomized trial.Trials. 2020 Jan 15;21(1):91. doi: 10.1186/s13063-019-4027-7. Trials. 2020. PMID: 31941527 Free PMC article. Clinical Trial.
-
Strategies for increasing participation in mail-out colorectal cancer screening programs: a systematic review and meta-analysis.Syst Rev. 2019 Nov 4;8(1):257. doi: 10.1186/s13643-019-1170-x. Syst Rev. 2019. PMID: 31685010 Free PMC article.
Cited by
-
Improving Smoking and Blood Pressure Outcomes: The Interplay Between Operational Changes and Local Context.Ann Fam Med. 2021 May-Jun;19(3):240-248. doi: 10.1370/afm.2668. Ann Fam Med. 2021. PMID: 34180844 Free PMC article.
-
What's the "secret sauce"? How implementation variation affects the success of colorectal cancer screening outreach.Implement Sci Commun. 2021 Jan 11;2(1):5. doi: 10.1186/s43058-020-00104-7. Implement Sci Commun. 2021. PMID: 33431063 Free PMC article.
-
Understanding Whether and How a Digital Health Intervention Improves Transition Care for Emerging Adults Living With Type 1 Diabetes: Protocol for a Mixed Methods Realist Evaluation.JMIR Res Protoc. 2023 Sep 13;12:e46115. doi: 10.2196/46115. JMIR Res Protoc. 2023. PMID: 37703070 Free PMC article.
-
How education and racial segregation intersect in neighborhoods with persistently low COVID-19 vaccination rates in Philadelphia.BMC Public Health. 2022 May 25;22(1):1044. doi: 10.1186/s12889-022-13414-3. BMC Public Health. 2022. PMID: 35614426 Free PMC article.
-
Barriers and proposed solutions to at-home colorectal cancer screening tests in medically underserved health centers across three US regions to inform a randomized trial.Cancer Med. 2024 Aug;13(15):e70040. doi: 10.1002/cam4.70040. Cancer Med. 2024. PMID: 39118261 Free PMC article.
References
-
- Davis MFM, Shannon J, Coronado G, Stange K, Guise JM, Wheeler S, Buckley DI. A systematic review of clinic and community intervention to increase fecal testing for colorectal cancer in rural and low-income populations in the United States – how, what and when?. BMC Cancer In Press. 2018. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials