Phosphorylation of NANOG by casein kinase I regulates embryonic stem cell self-renewal
- PMID: 33107035
- PMCID: PMC7839479
- DOI: 10.1002/1873-3468.13969
Phosphorylation of NANOG by casein kinase I regulates embryonic stem cell self-renewal
Abstract
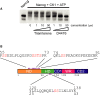
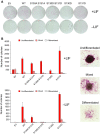
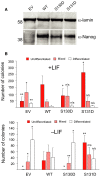
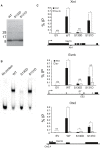
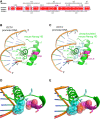
The self-renewal efficiency of mouse embryonic stem cells (ESCs) is determined by the concentration of the transcription factor NANOG. While NANOG binds thousands of sites in chromatin, the regulatory systems that control DNA binding are poorly characterised. Here, we show that NANOG is phosphorylated by casein kinase I, and identify target residues. Phosphomimetic substitutions at phosphorylation sites within the homeodomain (S130 and S131) have site-specific functional effects. Phosphomimetic substitution of S130 abolishes DNA binding by NANOG and eliminates LIF-independent self-renewal. In contrast, phosphomimetic substitution of S131 enhances LIF-independent self-renewal, without influencing DNA binding. Modelling the DNA-homeodomain complex explains the disparate effects of these phosphomimetic substitutions. These results indicate how phosphorylation may influence NANOG homeodomain interactions that underpin ESC self-renewal.
Keywords: DNA binding; NANOG; casein kinase I; phosphorylation; self-renewal.
© 2020 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures
References
-
- Das C and Kundu TK (2005) Transcriptional regulation by the acetylation of nonhistone proteins in humans – a new target for therapeutics. IUBMB Life 57, 137–149. - PubMed
-
- Hammond‐Martel I, Yu H and el Affar B (2012) Roles of ubiquitin signaling in transcription regulation. Cell Signal 24, 410–421. - PubMed
-
- Levy DE and Darnell JE Jr (2002) Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 3, 651–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/T003162/1/MRC_/Medical Research Council/United Kingdom
- BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/L018497/1/MRC_/Medical Research Council/United Kingdom
- MR/N000609/1/MRC_/Medical Research Council/United Kingdom
- MR/K017047/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

