SMARCA4 mutations in KRAS-mutant lung adenocarcinoma: a multi-cohort analysis
- PMID: 33107184
- PMCID: PMC7858279
- DOI: 10.1002/1878-0261.12831
SMARCA4 mutations in KRAS-mutant lung adenocarcinoma: a multi-cohort analysis
Abstract
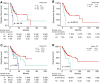
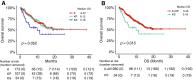
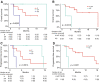
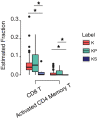
KRAS is a key oncogenic driver in lung adenocarcinoma (LUAD). Chromatin-remodeling gene SMARCA4 is comutated with KRAS in LUAD; however, the impact of SMARCA4 mutations on clinical outcome has not been adequately established. This study sought to shed light on the clinical significance of SMARCA4 mutations in LUAD. The association of SMARCA4 mutations with survival outcomes was interrogated in four independent cohorts totaling 564 patients: KRAS-mutant patients with LUAD who received nonimmunotherapy treatment from (a) The Cancer Genome Atlas (TCGA) and (b) the MSK-IMPACT Clinical Sequencing (MSK-CT) cohorts; and KRAS-mutant patients with LUAD who received immune checkpoint inhibitor-based immunotherapy treatment from (c) the MSK-IMPACT (MSK-IO) and (d) the Wake Forest Baptist Comprehensive Cancer Center (WFBCCC) immunotherapy cohorts. Of the patients receiving nonimmunotherapy treatment, in the TCGA cohort (n = 155), KRAS-mutant patients harboring SMARCA4 mutations (KS) showed poorer clinical outcome [P = 6e-04 for disease-free survival (DFS) and 0.031 for overall survival (OS), respectively], compared to KRAS-TP53 comutant (KP) and KRAS-only mutant (K) patients; in the MSK-CT cohort (n = 314), KS patients also exhibited shorter OS than KP (P = 0.03) or K (P = 0.022) patients. Of patients receiving immunotherapy, KS patients consistently exhibited the shortest progression-free survival (PFS; P = 0.0091) in the MSK-IO (n = 77), and the shortest PFS (P = 0.0026) and OS (P = 0.0014) in the WFBCCC (n = 18) cohorts, respectively. Therefore, mutations of SMARCA4 represent a genetic factor leading to adverse clinical outcome in lung adenocarcinoma treated by either nonimmunotherapy or immunotherapy.
Keywords: KRAS; SMARCA4 mutation; immunotherapy; lung adenocarcinoma; nonimmunotherapy; prognostics biomarker.
© 2020 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L et al (2015) Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 372, 2018–2028. - PubMed
-
- Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal‐Cortes A, Lewanski C et al (2016) Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): a multicentre, open‐label, phase 2 randomised controlled trial. Lancet 387, 1837–1846. - PubMed
-
- Herbst RS, Baas P, Kim DW, Felip E, Perez‐Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ et al (2016) Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet 387, 1540–1550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

