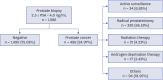
Strategy for Prostate Cancer Patients with Low Prostate Specific Antigen Level (2.5 to 4.0 ng/mL)
- PMID: 33107227
- PMCID: PMC7590649
- DOI: 10.3346/jkms.2020.35.e342
Strategy for Prostate Cancer Patients with Low Prostate Specific Antigen Level (2.5 to 4.0 ng/mL)
Abstract
Background: To evaluate the strategy for detection of prostate cancer (PCa) with low prostate specific antigen (PSA) level (2.5-4.0 ng/mL), prostate biopsy patients with low PSA were assessed. We evaluated the risk of low PSA PCa and the strategy for screening low-PSA patients.
Methods: We retrospectively analyzed the patients who underwent prostate biopsy with low PSA level. Baseline characteristics, PSA level before prostate biopsy, prostate volume, prostate specific antigen density (PSAD), and pathological data were assessed.
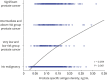
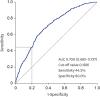
Results: Among the 1986 patients, 24.97% were diagnosed with PCa. The PSAD was 0.12 ± 0.04 ng/mL² in the PCa-diagnosed group and 0.10 ± 0.04 ng/mL² in non-cancer-diagnosed group (P < 0.001). Of the 496 patients diagnosed with PCa, 302 (60.89%) were in the intermediate- or high-risk group. PSAD was 0.13 ± 0.04 ng/mL² in the intermediate- or high-risk group and 0.11 ± 0.03 ng/mL² in the very low- and low-risk group (P < 0.001). Of 330 patients who underwent radical prostatectomy, 85.15% were diagnosed as having significant cancer. There was significant correlation between PSAD and PCa (r = 0.294, P < 0.001). PSAD with a specificity of 80.00% of a clinically significant cancer diagnosis was assessed at 0.1226 ng/mL².
Conclusion: The PCa detection rate in the low-PSA group was not lower than that of previous studies of patients with PSA from 4.0 to 10.0 ng/mL. Further, it may be helpful to define a strategy for PCa detection using PSAD in the low-PSA group.
Keywords: Biopsy; Diagnosis; Prostate-Specific Antigen; Prostatic Neoplasms.
© 2020 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures

References
-
- Verma A, St Onge J, Dhillon K, Chorneyko A. PSA density improves prediction of prostate cancer. Can J Urol. 2014;21(3):7312–7321. - PubMed
-
- Littrup PJ, Kane RA, Mettlin CJ, Murphy GP, Lee F, Toi A, et al. Cost-effective prostate cancer detection. Reduction of low-yield biopsies. Cancer. 1994;74(12):3146–3158. - PubMed
-
- Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA. 1997;277(18):1452–1455. - PubMed
-
- Schröder FH, van der Cruijsen-Koeter I, de Koning HJ, Vis AN, Hoedemaeker RF, Kranse R. Prostate cancer detection at low prostate specific antigen. J Urol. 2000;163(3):806–812. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

