Sarcomere length non-uniformities dictate force production along the descending limb of the force-length relation
- PMID: 33109011
- PMCID: PMC7661311
- DOI: 10.1098/rspb.2020.2133
Sarcomere length non-uniformities dictate force production along the descending limb of the force-length relation
Abstract
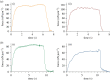
The force-length relation is one of the most defining features of muscle contraction, and yet a topic of debate in the literature. The sliding filament theory predicts that the force produced by muscle fibres is proportional to the degree of overlap between myosin and actin filaments, producing a linear descending limb of the active force-length relation. However, several studies have shown forces that are larger than predicted, especially at long sarcomere lengths (SLs). Studies have been conducted with muscle fibres, preparations containing thousands of sarcomeres that make measurements of individual SL challenging. The aim of this study was to evaluate force production and sarcomere dynamics in isolated myofibrils and single sarcomeres from the rabbit psoas muscle to enhance our understanding of the theoretically predicted force-length relation. Contractions at varying SLs along the plateau (SL = 2.25-2.39 µm) and the descending limb (SL > 2.39 µm) of the force-length relation were induced in sarcomeres and myofibrils, and different modes of force measurements were used. Our results show that when forces are measured in single sarcomeres, the experimental force-length relation follows theoretical predictions. When forces are measured in myofibrils with large SL dispersions, there is an extension of the plateau and forces elevated above the predicted levels along the descending limb. We also found an increase in SL non-uniformity and slowed rates of force production at long lengths in myofibrils but not in single sarcomere preparations. We conclude that the deviation of the descending limb of the force-length relation is correlated with the degree of SL non-uniformity and slowed force development.
Keywords: force–length relation; myofibril; sarcomere length non-uniformity; single sarcomere.
Conflict of interest statement
We declare we have no competing interests
Figures





References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources

