circCRAMP1L is a novel biomarker of preeclampsia risk and may play a role in preeclampsia pathogenesis via regulation of the MSP/RON axis in trophoblasts
- PMID: 33109096
- PMCID: PMC7590488
- DOI: 10.1186/s12884-020-03345-5
circCRAMP1L is a novel biomarker of preeclampsia risk and may play a role in preeclampsia pathogenesis via regulation of the MSP/RON axis in trophoblasts
Abstract
Background: Preeclampsia is a severe disease in pregnant women, which is primarily managed by early screening and prevention. Circular RNAs (circRNAs) have increasingly been shown to be important biological regulators involved in numerous diseases. Further, increasing evidence has demonstrated that circRNAs can be used as diagnostic biomarkers. This study was conducted to evaluate the potential of circCRAMP1L, previously identified to be downregulated in preeclampsia, as a novel biomarker for predicting the development of preeclampsia.
Methods: We measured the expression of circCRAMP1L, which is reportedly relevant to trophoblast physiology, in plasma samples from 64 patients with preeclampsia and 64 age-, gestational age-, and body mass index-matched healthy pregnant women by qRT-PCR. MTT proliferation and transwell invasion assays revealed the biological role of circCRAMP1L in preeclampsia pathogenesis. RNA immunoprecipitation and dual-luciferase reporter assays clarified the mechanism underlying the biological function of circCRAMP1L in TEV-1 cells.
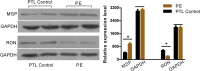
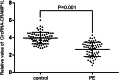
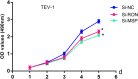
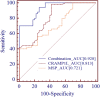
Results: circCRAMP1L circulating levels were significantly lower in patients with preeclampsia (2.66 ± 0.82, △Ct value) than in healthy pregnant women (3.95 ± 0.67, △Ct value, p < 0.001). The area under the receiver operating characteristic curve for circCRAMP1L was 0.813. Univariate and multivariate analyses identified circCRAMP1L as an independent predictor of preeclampsia. Furthermore, when circCRAMP1L was utilised in combination with its target protein macrophage stimulating protein (MSP), the predictive performance increased, with an area under the receiver operating characteristic curve of 0.928 (95% CI 0.882-0.974), 80.0% sensitivity, and 80.0% specificity. The in vitro results indicated that circCRAMP1L regulates cell proliferation, and invasion via MSP and RON proteins. We investigated the molecular mechanisms of these effects. In vitro, relative to the control group, circCRAMP1L overexpression significantly enhanced cell proliferation; furthermore, trophoblast cell invasion increased proportionally with circCRAMP1L expression. RNA immunoprecipitation and luciferase reporter gene illustrated that circCRAMP1L participated in regulation of trophoblast cell by regulating MSP.
Conclusion: Reduced plasma levels of circCRAMP1L may be associated with an increased risk of preeclampsia, and circCRAMP1L may be a novel biomarker of preeclampsia risk.
Keywords: MSP/RON; Preeclampsia; circCRAMP1L.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures







References
-
- Practice Bulletin No ACOG. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1–e25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81801474/the National Natural Science Foundation of China
- 81871716/the National Natural Science Foundation of China
- JCYJ20180306172502097/the Science and Technology Fund of Shenzhen
- 2017096/Science and technology special fund of Longhua District
- 201707010019/The Science and Technology Fund of Guangzhou
LinkOut - more resources
Full Text Sources
Research Materials

